Role of astrocytes and chemokine systems in acute TNFalpha induced demyelinating syndrome: CCR2-dependent signals promote astrocyte activation and survival via NF-kappaB and Akt
- PMID: 17949991
- PMCID: PMC2894699
- DOI: 10.1016/j.mcn.2007.08.017
Role of astrocytes and chemokine systems in acute TNFalpha induced demyelinating syndrome: CCR2-dependent signals promote astrocyte activation and survival via NF-kappaB and Akt
Abstract
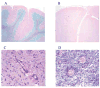
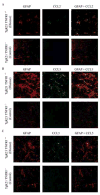
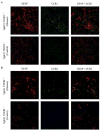
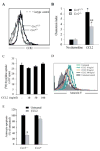
Chemotactic factors known as chemokines play an important role in the pathogenesis of multiple sclerosis (MS). Transgenic expression of TNFalpha in the central nervous system (CNS) leads to the development of a demyelinating phenotype (TNFalpha-induced demyelination; TID) that is highly reminiscent of MS. Little is known about the role of chemokines in TID but insights derived from studying this model might extend our current understanding of MS pathogenesis and complement data derived from the classic autoimmune encephalomyelitis (EAE) model system. Here we show that in TID, chemokines and their receptors were significantly increased during the acute phases of disease. Notably, the CCL2 (MCP-1)-CCR2 axis and the closely related ligand-receptor pair CCR1-CCL3 (MIP-1alpha) were among the most up-regulated during disease. On the other hand, receptors like CCR3 and CCR4 were not elevated. This significant increase in the levels of chemokines/receptors correlated with robust immune infiltration of the CNS by inflammatory cells, i.e., macrophages, and immune cells particularly T and B cells. Immunostaining and confocal microscopy, along with in vitro studies revealed that astrocytes were a major source of locally produced chemokines and expressed functional chemokine receptors such as CCR2. Using an in vitro system we demonstrate that expression of CCR2 was functional in astrocytes and that signaling via this receptor lead to activation of NF-kB and Akt and was associated with increased astrocyte survival. Collectively, our data suggests that transgenic murine models of MS are useful to dissect mechanisms of disease and that in these models, up-regulation of chemokines and their receptors may be key determinants in TID.
Figures

Similar articles
-
Chemokines in the MPTP model of Parkinson's disease: absence of CCL2 and its receptor CCR2 does not protect against striatal neurodegeneration.Brain Res. 2007 Jan 12;1128(1):1-11. doi: 10.1016/j.brainres.2006.08.041. Epub 2006 Nov 28. Brain Res. 2007. PMID: 17126305
-
Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones.J Neurochem. 2005 Nov;95(4):1023-34. doi: 10.1111/j.1471-4159.2005.03431.x. Epub 2005 Sep 7. J Neurochem. 2005. PMID: 16150057
-
Glial fibrillary acidic protein expression alters astrocytic chemokine release and protects mice from cuprizone-induced demyelination.Glia. 2019 Jul;67(7):1308-1319. doi: 10.1002/glia.23605. Epub 2019 Feb 23. Glia. 2019. PMID: 30801815
-
The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE).Semin Immunol. 2003 Feb;15(1):23-32. doi: 10.1016/s1044-5323(02)00125-2. Semin Immunol. 2003. PMID: 12495638 Review.
-
Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks.J Cereb Blood Flow Metab. 2010 Mar;30(3):459-73. doi: 10.1038/jcbfm.2009.240. Epub 2009 Nov 11. J Cereb Blood Flow Metab. 2010. PMID: 19904283 Free PMC article. Review.
Cited by
-
Absence of CCL2 and CCL3 Ameliorates Central Nervous System Grey Matter But Not White Matter Demyelination in the Presence of an Intact Blood-Brain Barrier.Mol Neurobiol. 2016 Apr;53(3):1551-1564. doi: 10.1007/s12035-015-9113-6. Epub 2015 Feb 8. Mol Neurobiol. 2016. PMID: 25663168
-
Overexpression of microRNA-381-3p ameliorates hypoxia/ischemia-induced neuronal damage and microglial inflammation via regulating the C-C chemokine receptor type 2 /nuclear transcription factor-kappa B axis.Bioengineered. 2022 Mar;13(3):6839-6855. doi: 10.1080/21655979.2022.2038448. Bioengineered. 2022. PMID: 35246016 Free PMC article.
-
Kit (W-sh) mice develop earlier and more severe experimental autoimmune encephalomyelitis due to absence of immune suppression.J Immunol. 2011 Jul 1;187(1):274-82. doi: 10.4049/jimmunol.1003603. Epub 2011 Jun 6. J Immunol. 2011. PMID: 21646293 Free PMC article.
-
GAS reduced inflammatory responses in activated microglia by regulating the Ccr2/Akt/Gsk-3β pathway.Mol Brain. 2025 May 6;18(1):40. doi: 10.1186/s13041-025-01206-w. Mol Brain. 2025. PMID: 40329396 Free PMC article.
-
Involvement of spinal chemokine CCL2 in the hyperalgesia evoked by bone cancer in mice: a role for astroglia and microglia.Cell Mol Neurobiol. 2014 Jan;34(1):143-56. doi: 10.1007/s10571-013-9995-7. Cell Mol Neurobiol. 2014. PMID: 24122510 Free PMC article.
References
-
- Akassoglou K, Adams RA, Bauer J, Mercado P, Tseveleki V, Lassmann H, Probert L, Strickland S. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101:6698–6703. - PMC - PubMed
-
- Akassoglou K, Bauer J, Kassiotis G, Lassmann H, Kollias G, Probert L. Transgenic models of TNF induced demyelination. Adv Exp Med Biol. 1999;468:245–259. - PubMed
-
- Akassoglou K, Bauer J, Kassiotis G, Pasparakis M, Lassmann H, Kollias G, Probert L. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy. Am J Pathol. 1998;153:801–813. - PMC - PubMed
-
- Akassoglou K, Probert L, Kontogeorgos G, Kollias G. Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J Immunol. 1997;158:438–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

