Activation of the nuclear factor of activated T-cells (NFAT) mediates upregulation of CCR2 chemokine receptors in dorsal root ganglion (DRG) neurons: a possible mechanism for activity-dependent transcription in DRG neurons in association with neuropathic pain
- PMID: 17949992
- PMCID: PMC2376051
- DOI: 10.1016/j.mcn.2007.09.004
Activation of the nuclear factor of activated T-cells (NFAT) mediates upregulation of CCR2 chemokine receptors in dorsal root ganglion (DRG) neurons: a possible mechanism for activity-dependent transcription in DRG neurons in association with neuropathic pain
Abstract
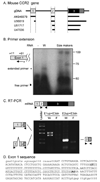
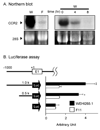
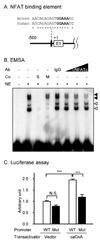
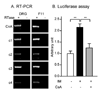
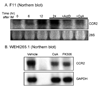
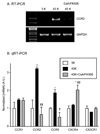
Upregulation of CCR2 chemokine receptor expression by dorsal root ganglion (DRG) neurons is an important process in the development and maintenance of neuropathic pain. CCR2 is not expressed by DRG neurons under normal conditions but is upregulated in several animal models of neuropathic pain where its signaling is excitatory. However, the molecular mechanisms underlying neuronal upregulation of CCR2 have not been investigated. We examined the promoter region of the CCR2 gene and found that a binding site for the nuclear factor of activated T-cells (NFAT) was conserved among species. The NFAT element was functional since the CCR2 promoter was activated by a constitutively active form of calcineurin A, whereas a point mutation in the NFAT binding site abrogated it. Activation of the NFAT pathway in the DRG neuronal cell line F11 increased CCR2 promoter activity and induced CCR2 transcription. Moreover, depolarization of cultured DRG neurons induced de novo synthesis of CCR2 mRNA, which was blocked by the calcineurin inhibitors cyclosporin A and FK506. These data indicate that CCR2 is a target of the NFAT pathway and suggest that tonic excitation of DRG neurons in association with chronic pain may lead to neuronal CCR2 upregulation via activation of the NFAT pathway.
Figures
Similar articles
-
Human Mas-related G protein-coupled receptors-X1 induce chemokine receptor 2 expression in rat dorsal root ganglia neurons and release of chemokine ligand 2 from the human LAD-2 mast cell line.PLoS One. 2013;8(3):e58756. doi: 10.1371/journal.pone.0058756. Epub 2013 Mar 7. PLoS One. 2013. PMID: 23505557 Free PMC article.
-
Oxaliplatin Regulates Chemotherapy Induced Peripheral Neuropathic Pain in the Dorsal Horn and Dorsal Root Ganglion via the Calcineurin/NFAT Pathway.Anticancer Agents Med Chem. 2018;18(8):1197-1207. doi: 10.2174/1871520618666180525091158. Anticancer Agents Med Chem. 2018. PMID: 29793414
-
Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons.J Neurochem. 2008 Jan;104(1):254-63. doi: 10.1111/j.1471-4159.2007.04969.x. Epub 2007 Oct 18. J Neurochem. 2008. PMID: 17944871 Free PMC article.
-
Nuclear factor of activated T cells and serum response factor cooperatively regulate the activity of an alpha-actin intronic enhancer.J Biol Chem. 2005 Jul 15;280(28):26113-20. doi: 10.1074/jbc.M411972200. Epub 2005 Apr 27. J Biol Chem. 2005. PMID: 15857835
-
Chemokine signaling and the management of neuropathic pain.Mol Interv. 2009 Aug;9(4):188-95. doi: 10.1124/mi.9.4.7. Mol Interv. 2009. PMID: 19720751 Free PMC article. Review.
Cited by
-
CC chemokine ligand 2 upregulates the current density and expression of TRPV1 channels and Nav1.8 sodium channels in dorsal root ganglion neurons.J Neuroinflammation. 2012 Aug 8;9:189. doi: 10.1186/1742-2094-9-189. J Neuroinflammation. 2012. PMID: 22870919 Free PMC article.
-
Potential Local Mechanisms for Exercise-Induced Hypoalgesia in Response to Blood Flow Restriction Training.Cureus. 2023 Aug 9;15(8):e43219. doi: 10.7759/cureus.43219. eCollection 2023 Aug. Cureus. 2023. PMID: 37692724 Free PMC article. Review.
-
Mrgprs activation is required for chronic itch conditions in mice.Itch (Phila). 2017 Dec;2(3):e9. doi: 10.1097/itx.0000000000000009. Itch (Phila). 2017. PMID: 29577089 Free PMC article.
-
Chemokines and the pathophysiology of neuropathic pain.Proc Natl Acad Sci U S A. 2007 Dec 18;104(51):20151-8. doi: 10.1073/pnas.0709250104. Epub 2007 Dec 14. Proc Natl Acad Sci U S A. 2007. PMID: 18083844 Free PMC article. Review.
-
Visualization of chemokine receptor activation in transgenic mice reveals peripheral activation of CCR2 receptors in states of neuropathic pain.J Neurosci. 2009 Jun 24;29(25):8051-62. doi: 10.1523/JNEUROSCI.0485-09.2009. J Neurosci. 2009. PMID: 19553445 Free PMC article.
References
-
- Basbaum AI, Woolf CJ. Pain. Curr Biol. 1999;9:R429–R431. - PubMed
-
- Bhangoo SK, Jung H, Chan DM, Ripsch M, Miller RJ, White FA. Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience, 2006; 2006. Peripheral demyelination injury induces upregulation of chemokine/receptor expression and neuronal signaling in a model of neuropathic pain. Online. 250.3.
-
- Clipstone NA, Fiorentino DF, Crabtree GR. Molecular analysis of the interaction of calcineurin with drug-immunophilin complexes. J Biol Chem. 1994;269:26431–26437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

