Cellular edema regulates tissue capillary perfusion after hemorrhage resuscitation
- PMID: 17950340
- PMCID: PMC2131728
- DOI: 10.1016/j.surg.2007.08.007
Cellular edema regulates tissue capillary perfusion after hemorrhage resuscitation
Erratum in
- Surgery. 2008 Feb;143(2):301
Abstract
Background: Hemorrhage-induced activation of endothelial cell Na+/H+ -exchanger results in cellular swelling, which physically impedes capillary filling and compromises gut perfusion. We hypothesized that correction of the vascular volume deficit by conventional resuscitation does not improve capillary filling unless cellular swelling is prevented. Also, we hypothesized that adjunctive direct peritoneal resuscitation (DPR) with topical peritoneal dialysis solution (Delflex; Fresenius USA, Inc., Ogden, Ut) enhances capillary filling and gut perfusion by mechanisms that are independent of the Na+/H+ function.
Methods: In vivo intravital videomicroscopy and Doppler velocimeter were used by us to measure microvascular diameter and flow, capillary filling (index of functional capillary density, FCD), and endothelial cell function in the terminal ileum of anesthetized rats. Rats were bled to 50% mean arterial pressure for 60 min and resuscitated with the shed blood plus 2 volumes of saline (conventional resuscitation). Prevention of endothelial cell swelling was achieved with topical amiloride (specific Na+/H+ inhibitor) in the tissue bath before hemorrhage or simultaneously with conventional resuscitation. DPR was simulated by instillation of Delflex in the tissue bath as adjunctive to conventional resuscitation. Sham no hemorrhage group and a simulated DPR group that received topical amiloride treatment served as controls.
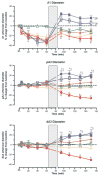
Results: Conventional resuscitation from hemorrhagic shock restored and maintained central hemodynamics but caused progressive and persistent intestinal vasoconstriction and hypoperfusion associated with low FCD and endothelial cell dysfunction. Prevention of endothelial cell swelling when combined with conventional resuscitation, preserved endothelial cell function, and restored local intestinal microvascular variables to near-prehemorrhage levels. Simulated adjunctive DPR produced rapid, sustained, and generalized vasodilation associated with restoration of endothelial cell function, and maximum recruitment of FCD independent of the Na+/H+ -exchanger function.
Conclusions: Paradoxical endothelial cell swelling occurs early during hemorrhagic shock because of activation of the Na+/H+ exchanger. This cellular edema, which is not resolved by correction of the vascular volume deficit, explains the persistent postresuscitation endothelial cell dysfunction and gut hypoperfusion. Simulated adjunctive DPR in this study reversed endothelial cell swelling and enhanced gut perfusion by mechanisms that are independent of the Na+/H+ exchanger activity.
Figures



Similar articles
-
Direct peritoneal resuscitation from hemorrhagic shock: effect of time delay in therapy initiation.J Trauma. 2005 Mar;58(3):499-506; discussion 506-8. doi: 10.1097/01.ta.0000152892.24841.54. J Trauma. 2005. PMID: 15761343 Free PMC article.
-
Mechanisms of direct peritoneal resuscitation-mediated splanchnic hyperperfusion following hemorrhagic shock.Shock. 2007 Apr;27(4):436-42. doi: 10.1097/01.shk.0000245017.86117.4e. Shock. 2007. PMID: 17414428 Free PMC article.
-
Plasma resuscitation with adjunctive peritoneal resuscitation reduces ischemic intestinal injury following hemorrhagic shock.J Trauma Acute Care Surg. 2020 Oct;89(4):649-657. doi: 10.1097/TA.0000000000002847. J Trauma Acute Care Surg. 2020. PMID: 32773670
-
Peritoneal resuscitation.Am J Surg. 2005 Aug;190(2):181-5. doi: 10.1016/j.amjsurg.2005.05.008. Am J Surg. 2005. PMID: 16023427 Review.
-
Hemorrhagic shock.Curr Probl Surg. 1995 Nov;32(11):925-1002. doi: 10.1016/s0011-3840(05)80008-5. Curr Probl Surg. 1995. PMID: 7587344 Review.
Cited by
-
Direct peritoneal resuscitation accelerates primary abdominal wall closure after damage control surgery.J Am Coll Surg. 2010 May;210(5):658-64, 664-7. doi: 10.1016/j.jamcollsurg.2010.01.014. J Am Coll Surg. 2010. PMID: 20421025 Free PMC article.
-
Resuscitation-induced intestinal edema and related dysfunction: state of the science.J Surg Res. 2011 Mar;166(1):120-30. doi: 10.1016/j.jss.2009.09.010. Epub 2009 Sep 29. J Surg Res. 2011. PMID: 19959186 Free PMC article. Review.
-
Cell Impermeant-based Low-volume Resuscitation in Hemorrhagic Shock: A Biological Basis for Injury Involving Cell Swelling.Ann Surg. 2016 Mar;263(3):565-72. doi: 10.1097/SLA.0000000000001049. Ann Surg. 2016. PMID: 25915911 Free PMC article.
-
Hemorrhage-induced hepatic injury and hypoperfusion can be prevented by direct peritoneal resuscitation.J Gastrointest Surg. 2009 Apr;13(4):587-94. doi: 10.1007/s11605-008-0796-0. Epub 2009 Jan 31. J Gastrointest Surg. 2009. PMID: 19184613 Free PMC article.
-
Hemorrhagic shock and resuscitation-mediated tissue water distribution is normalized by adjunctive peritoneal resuscitation.J Am Coll Surg. 2008 May;206(5):970-80; discussion 980-3. doi: 10.1016/j.jamcollsurg.2007.12.035. Epub 2008 Mar 24. J Am Coll Surg. 2008. PMID: 18471737 Free PMC article.
References
-
- Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1–10. - PubMed
-
- Mazzoni MC, Intaglietta M, Cragoe EJ, Jr, Arfors KE. Amiloride-sensitive Na+ pathways in capillary endothelial cell swelling during hemorrhagic shock. J Appl Physiol. 1992;73:1467–73. - PubMed
-
- Chaudry IH, Baue A. Alterations in adenosine 3′,5′-monophosphate levels in hemorrhagic shock. Surg Forum. 1976;27:51–3. - PubMed
-
- Chaudry IH, Sayeed MM, Baue AE. Alterations in high-energy phosphates in hemorrhagic shock as related to tissue and organ function. Surgery. 1976;79:666–8. - PubMed
-
- Van WC, III, Dhar A, Morrison DC, Longorio MA, Maxfield DM. Cellular energetics in hemorrhagic shock: restoring adenosine triphosphate to the cells. J Trauma. 2003;54:S169–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

