Decreased substance P and NK1 receptor immunoreactivity and function in the spinal cord dorsal horn of morphine-treated neonatal rats
- PMID: 17950674
- PMCID: PMC2241645
- DOI: 10.1016/j.jpain.2007.07.008
Decreased substance P and NK1 receptor immunoreactivity and function in the spinal cord dorsal horn of morphine-treated neonatal rats
Abstract
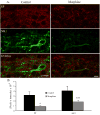
Opiate analgesic tolerance is defined as a need for higher doses of opiates to maintain pain relief after prolonged opiate exposure. Though changes in the opioid receptor undoubtedly occur during conditions of opiate tolerance, there is increasing evidence that opiate analgesic tolerance is also caused by pronociceptive adaptations in the spinal cord. We have previously observed increased glutamate release in the spinal cord dorsal horn of neonatal rats made tolerant to the opiate morphine. In this study, we investigate whether spinal substance P (SP) and its receptor, the neurokinin 1 (NK1) receptor, are also modulated by prolonged morphine exposure. Immunocytochemical studies show decreased SP- and NK1-immunoreactivity in the dorsal horn of morphine-treated rats, whereas SP mRNA in the dorsal root ganglia is not changed. Electrophysiological studies show that SP fails to activate the NK1 receptor in the morphine-treated rat. Taken together, the data indicate that chronic morphine treatment in the neonatal rat is characterized by a loss of SP effects on the NK1 receptor in lamina I of the neonatal spinal cord dorsal horn. The results are discussed in terms of compensatory spinal cord processes that may contribute to opiate analgesic tolerance.
Perspective: This article describes anatomical and physiological changes that occur in the spinal cord dorsal horn of neonatal rats after chronic morphine treatment. These changes may represent an additional compensatory process of morphine tolerance and may represent an additional therapeutic target for the retention and restoration of pain relief with prolonged morphine treatment.
Figures



References
-
- Abbadie C, Brown JL, Mantyh PW, Basbaum AI. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–9. - PubMed
-
- Afrah AW, Fiska A, Gjerstad J, Gustafsson H, Tjolsen A, Olgart L, Stiller CO, Hole K, Brodin E. Spinal substance P release in vivo during the induction of long-term potentiation in dorsal horn neurons. Pain. 2002;96:49–55. - PubMed
-
- Belanger S, Ma W, Chabot JG, Quirion R. Expression of calcitonin gene-related peptide, substance P and protein kinase C in cultured dorsal root ganglion neurons following chronic exposure to mu, delta and kappa opiates. Neuroscience. 2002;115:441–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

