Oral supplementation with docosahexaenoic acid and uridine-5'-monophosphate increases dendritic spine density in adult gerbil hippocampus
- PMID: 17950710
- PMCID: PMC2140951
- DOI: 10.1016/j.brainres.2007.08.089
Oral supplementation with docosahexaenoic acid and uridine-5'-monophosphate increases dendritic spine density in adult gerbil hippocampus
Abstract
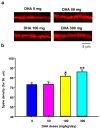
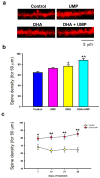
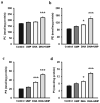
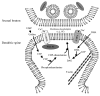
Docosahexaenoic acid (DHA), an omega-3 polyunsaturated fatty acid, is an essential component of membrane phosphatides and has been implicated in cognitive functions. Low levels of circulating or brain DHA are associated with various neurocognitive disorders including Alzheimer's disease (AD), while laboratory animals, including animal models of AD, can exhibit improved cognitive ability with a diet enriched in DHA. Various cellular mechanisms have been proposed for DHA's behavioral effects, including increases in cellular membrane fluidity, promotion of neurite extension and inhibition of apoptosis. However, there is little direct evidence that DHA affects synaptic structure in living animals. Here we show that oral supplementation with DHA substantially increases the number of dendritic spines in adult gerbil hippocampus, particularly when animals are co-supplemented with a uridine source, uridine-5'-monophosphate (UMP), which increases brain levels of the rate-limiting phosphatide precursor CTP. The increase in dendritic spines (>30%) is accompanied by parallel increases in membrane phosphatides and in pre- and post-synaptic proteins within the hippocampus. Hence, oral DHA may promote neuronal membrane synthesis to increase the number of synapses, particularly when co-administered with UMP. Our findings provide a possible explanation for the effects of DHA on behavior and also suggest a strategy to treat cognitive disorders resulting from synapse loss.
Figures


References
-
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. - PMC - PubMed
-
- Appleton KM, Hayward RC, Gunnel D, Peters TJ, Rogers PJ, Kessler D, Ness AR. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials. Am J Clin Nutr. 2006;84:1308–1316. - PubMed
-
- Assies J, Lieverse R, Vreken P, Wanders RJ, Dingemans PM, Linszen DH. Significantly reduced docosahexaenoic and docosapentaenoic acid concentrations in erythrocyte membranes from schizophrenic patients compared with a carefully matched control group. Biol Psychiatry. 2001;49:510–522. - PubMed
-
- Blanpied TA, Ehlers MD. Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry. 2004;55:1121–1127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

