Pathophysiology and fate of hepatocytes in a mouse model of mitochondrial hepatopathies
- PMID: 17951359
- PMCID: PMC2730640
- DOI: 10.1136/gut.2006.119180
Pathophysiology and fate of hepatocytes in a mouse model of mitochondrial hepatopathies
Abstract
Background: Although oxidative phosphorylation defects can affect the liver, these conditions are poorly understood, partially because of the lack of animal models.
Aims: To create and characterise the pathophysiology of mitochondrial hepatopathies in a mouse model.
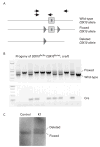
Methods: A mouse model of mitochondrial hepatopathies was created by the conditional liver knockout (KO) of the COX10 gene, which is required for cytochrome c oxidase (COX) function. The onset and progression of biochemical, molecular and clinical phenotypes were analysed in several groups of animals, mostly at postnatal days 23, 56, 78 and 155.
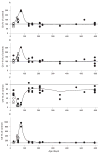
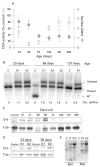
Results: Biochemical and histochemical analysis of liver samples from 23-56-day-old KO mice showed liver dysfunction, a severe COX deficiency, marked mitochondrial proliferation and lipid accumulation. Despite these defects, the COX-deficient hepatocytes were not immediately eliminated, and apoptosis followed by liver regeneration could be observed only at age 78 days. Hepatocytes from 56-78-day-old KO mice survived despite very low COX activity but showed a progressive depletion of glycogen stores. In most animals, hepatocytes that escaped COX10 ablation were able to proliferate and completely regenerate the liver between days 78 and 155.
Conclusions: The results showed that when faced with a severe oxidative phosphorylation defect, hepatocytes in vivo can rely on glycolysis/glycogenolysis for their bioenergetic needs for relatively long periods. Ultimately, defective hepatocytes undergo apoptosis and are replaced by COX-positive cells first observed in the perivascular regions.
Figures





References
-
- Chinnery PF, DiMauro S. Mitochondrial hepatopathies. J Hepatol. 2005;43:207–9. - PubMed
-
- Bandyopadhyay SK, Dutta A. Mitochondrial hepatopathies. J Assoc Physicians India. 2005;53:973–8. - PubMed
-
- Sokol RJ, Treem WR. Mitochondria and childhood liver diseases. J Pediatr Gastroenterol Nutr. 1999;28:4–16. - PubMed
-
- Anderson S, Bankier AT, Barrell BG, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–65. - PubMed
-
- Tsukihara T, Aoyama H, Yamashita E, et al. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å. Science. 1996;272:1136–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
