Genome-wide transcriptional profiling of the cyclic AMP-dependent signaling pathway during morphogenic transitions of Candida albicans
- PMID: 17951520
- PMCID: PMC2168245
- DOI: 10.1128/EC.00318-07
Genome-wide transcriptional profiling of the cyclic AMP-dependent signaling pathway during morphogenic transitions of Candida albicans
Abstract
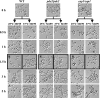
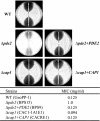
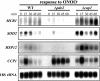
Candida albicans is an opportunistic human fungal pathogen that causes systemic candidiasis as well as superficial mucosal candidiasis. In response to the host environment, C. albicans transitions between yeast and hyphal forms. In particular, hyphal growth is important in facilitating adhesion and invasion of host tissues, concomitant with the expression of various hypha-specific virulence factors. In previous work, we showed that the cyclic AMP (cAMP) signaling pathway plays a crucial role in morphogenic transitions and virulence of C. albicans by studying genes encoding adenylate cyclase-associated protein (CAP1) and high-affinity phosphodiesterase (PDE2) (Y. S. Bahn, J. Staab, and P. Sundstrom, Mol. Microbiol. 50:391-409, 2003; and Y. S. Bahn and P. Sundstrom, J. Bacteriol. 183:3211-3223, 2001). However, little is known about the downstream targets of the cAMP signaling pathway that are responsible for morphological transitions and the expression of virulence factors. Here, microarrays were probed with RNA from strains with hypoactive (cap1/cap1 null mutant), hyperactive (pde2/pde2 null mutant), and wild-type cAMP signaling pathways to provide insight into the molecular mechanisms of virulence that are regulated by cAMP and that are related to the morphogenesis of C. albicans. Genes controlling metabolic specialization, cell wall structure, ergosterol/lipid biosynthesis, and stress responses were modulated by cAMP during hypha formation. Phenotypic traits predicted to be regulated by cAMP from the profiling results correlated with the relative strengths of the mutants when tested for resistance to azoles and subjected to heat shock stress and oxidative/nitrosative stress. The results from this study provide important insights into the role of the cAMP signaling pathway not only in morphogenic transitions of C. albicans but also for adaptation to stress and for survival during host infections.
Figures




References
-
- Alspaugh, J. A., R. Pukkila-Worley, T. Harashima, L. M. Cavallo, D. Funnell, G. M. Cox, J. R. Perfect, J. W. Kronstad, and J. Heitman. 2002. Adenylyl cyclase functions downstream of the Gα protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell 1:75-84. - PMC - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1994. Current protocols in molecular biology. Greene Publishing Associates and John Wiley & Sons, New York, NY.
-
- Bahn, Y. S., J. Staab, and P. Sundstrom. 2003. Increased high-affinity phosphodiesterase PDE2 gene expression in germ tubes counteracts CAP1-dependent synthesis of cyclic AMP, limits hypha production and promotes virulence of Candida albicans. Mol. Microbiol. 50:391-409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

