Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium
- PMID: 17954405
- PMCID: PMC2121222
- DOI: 10.1016/j.hrthm.2007.07.012
Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium
Abstract
Background: Styryl voltage-sensitive dyes (e.g., di-4-ANEPPS) have been used successfully for optical mapping in cardiac cells and tissues. However, their utility for probing electrical activity deep inside the myocardial wall and in blood-perfused myocardium has been limited because of light scattering and high absorption by endogenous chromophores and hemoglobin at blue-green excitation wavelengths.
Objective: The purpose of this study was to characterize two new styryl dyes--di-4-ANBDQPQ (JPW-6003) and di-4-ANBDQBS (JPW-6033)--optimized for blood-perfused tissue and intramural optical mapping.
Methods: Voltage-dependent spectra were recorded in a model lipid bilayer. Optical mapping experiments were conducted in four species (mouse, rat, guinea pig, and pig). Hearts were Langendorff perfused using Tyrode's solution and blood (pig). Dyes were loaded via bolus injection into perfusate. Transillumination experiments were conducted in isolated coronary-perfused pig right ventricular wall preparations.
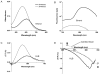
Results: The optimal excitation wavelength in cardiac tissues (650 nm) was >70 nm beyond the absorption maximum of hemoglobin. Voltage sensitivity of both dyes was approximately 10% to 20%. Signal decay half-life due to dye internalization was 80 to 210 minutes, which is 5 to 7 times slower than for di-4-ANEPPS. In transillumination mode, DeltaF/F was as high as 20%. In blood-perfused tissues, DeltaF/F reached 5.5% (1.8 times higher than for di-4-ANEPPS).
Conclusion: We have synthesized and characterized two new near-infrared dyes with excitation/emission wavelengths shifted >100 nm to the red. They provide both high voltage sensitivity and 5 to 7 times slower internalization rate compared to conventional dyes. The dyes are optimized for deeper tissue probing and optical mapping of blood-perfused tissue, but they also can be used for conventional applications.
Figures

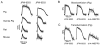
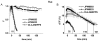
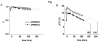

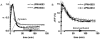

References
-
- Jalife J. Ventricular fibrillation: mechanisms of initiation maintenance. Annu Rev Physiol. 2000;62:25–50. - PubMed
-
- Fast V, Kleber A. Microscopic conduction in cultured strands of neonatal rat heart cells measured with voltage-sensitive dyes. Circ Res. 1993;73:914–25. - PubMed
-
- Khait VD, Bernus O, Mironov SF, Pertsov AM. Method for the three-dimensional localization of intramyocardial excitation centers using optical imaging. J Biomed Opt. 2006;11:34007. - PubMed
-
- Kalifa J, Klos M, Zlochiver S, Mironov S, Tanaka K, Ulahannan N, Yamazaki M, Jalife J, Berenfeld O. Endoscopic fluorescence mapping of the left atrium: A novel experimental approach for high resolution endocardial mapping in the intact heart. Heart Rhythm. 2007 doi: 10.1016/j.hrthm.2007.03.009. Epub 2007 March 16. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

