The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development
- PMID: 17954558
- PMCID: PMC2223292
- DOI: 10.1128/MCB.01119-07
The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development
Abstract
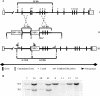
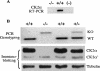
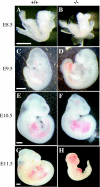
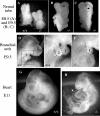
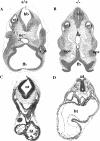
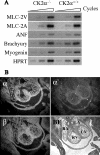
Protein kinase CK2 (formerly casein kinase II) is a highly conserved and ubiquitous serine/threonine kinase that is composed of two catalytic subunits (CK2alpha and/or CK2alpha') and two CK2beta regulatory subunits. CK2 has many substrates in cells, and key roles in yeast cell physiology have been uncovered by introducing subunit mutations. Gene-targeting experiments have demonstrated that in mice, the CK2beta gene is required for early embryonic development, while the CK2alpha' subunit appears to be essential only for normal spermatogenesis. We have used homologous recombination to disrupt the CK2alpha gene in the mouse germ line. Embryos lacking CK2alpha have a marked reduction in CK2 activity in spite of the presence of the CK2alpha' subunit. CK2alpha(-/-) embryos die in mid-gestation, with abnormalities including open neural tubes and reductions in the branchial arches. Defects in the formation of the heart lead to hydrops fetalis and are likely the cause of embryonic lethality. Thus, CK2alpha appears to play an essential and uncompensated role in mammalian development.
Figures
References
-
- Ackermann, K., A. Waxmann, C. V. Glover, and W. Pyerin. 2001. Genes targeted by protein kinase CK2: a genome-wide expression array analysis in yeast. Mol. Cell. Biochem. 22759-66. - PubMed
-
- Bidwai, A. P., J. C. Reed, and C. V. Glover. 1995. Cloning and disruption of CKB1, the gene encoding the 38-kDa beta subunit of Saccharomyces cerevisiae casein kinase II (CKII). Deletion of CKII regulatory subunits elicits a salt-sensitive phenotype. J. Biol. Chem. 27010395-10404. - PubMed
-
- Bosc, D. G., K. C. Graham, R. B. Saulnier, C. Zhang, D. Prober, R. D. Gietz, and D. W. Litchfield. 2000. Identification and characterization of CKIP-1, a novel pleckstrin homology domain-containing protein that interacts with protein kinase CK2. J. Biol. Chem. 27514295-14306. - PubMed
-
- Buchou, T., M. Vernet, O. Blond, H. H. Jensen, H. Pointu, B. B. Olsen, C. Cochet, O. G. Issinger, and B. Boldyreff. 2003. Disruption of the regulatory beta subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol. Cell. Biol. 23908-915. - PMC - PubMed
-
- Channavajhala, P., and D. C. Seldin. 2002. Functional interaction of protein kinase CK2 and c-Myc in lymphomagenesis. Oncogene 215280-5288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
