Determination of ubidecarenone (coenzyme Q10, ubiquinol-10) in raw materials and dietary supplements by high-performance liquid chromatography with ultraviolet detection: single-laboratory validation
- PMID: 17955966
- PMCID: PMC2586112
Determination of ubidecarenone (coenzyme Q10, ubiquinol-10) in raw materials and dietary supplements by high-performance liquid chromatography with ultraviolet detection: single-laboratory validation
Abstract
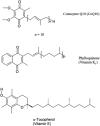
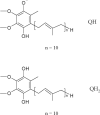
A method based on high-performance liquid chromatography with ultraviolet detection has been developed to quantify ubidecarenone [coenzyme Q10 (CoQ10)] in raw materials and dietary supplements. Single-laboratory validation has been performed on the method to determine repeatability, accuracy, selectivity, limits of detection and quantification (LOQ), ruggedness, and linearity for CoQ10. As CoQ10 can exist as the biologically active reduced form, the application of an oxidizing agent, ferric chloride, drives the equilibrium mechanics to the fully oxidized state and allows for exact quantification of total CoQ10 in the sample. This method was found to be fit and linear for the testing of materials containing CoQ10 in the range of approximately equal 50-1000 mg/g. Repeatability precision for CoQ10 was between 2.15 and 5.00% relative standard deviation. Observed recovery of CoQ10 was found to be between 93.8 and 100.9%. LOQ was found to be 9 microg/mL. Further, limited studies showed that some adulterants and degraded material could be satisfactorily separated from CoQ10 and identified.
Figures







Similar articles
-
Determination of coenzyme Q10 content in raw materials and dietary supplements by high-performance liquid chromatography-UV: collaborative study.J AOAC Int. 2008 Jul-Aug;91(4):702-8. J AOAC Int. 2008. PMID: 18727527 Free PMC article.
-
Determination of coenzyme Q10 in over-the-counter dietary supplements by high-performance liquid chromatography with coulometric detection.J AOAC Int. 2006 Jan-Feb;89(1):35-9. J AOAC Int. 2006. PMID: 16512225
-
Analysis of coenzyme Q(10) in human plasma by column-switching liquid chromatography.J Chromatogr B Analyt Technol Biomed Life Sci. 2004 Jun 15;805(2):297-301. doi: 10.1016/j.jchromb.2004.03.008. J Chromatogr B Analyt Technol Biomed Life Sci. 2004. PMID: 15135104
-
Coenzyme Q10 analytical determination in biological matrices and pharmaceuticals.Front Biosci (Schol Ed). 2016 Jun 1;8(2):321-30. doi: 10.2741/s466. Front Biosci (Schol Ed). 2016. PMID: 27100710 Review.
-
Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations.Mitochondrion. 2007 Jun;7 Suppl:S78-88. doi: 10.1016/j.mito.2007.03.003. Epub 2007 Mar 27. Mitochondrion. 2007. PMID: 17482886 Review.
Cited by
-
Cultivation at high osmotic pressure confers ubiquinone 8-independent protection of respiration on Escherichia coli.J Biol Chem. 2020 Jan 24;295(4):981-993. doi: 10.1074/jbc.RA119.011549. Epub 2019 Dec 11. J Biol Chem. 2020. PMID: 31826918 Free PMC article.
-
Preparation and Evaluation of Complexed Ubiquinone (Coenzyme Q10) Antiaging Hyaluronic Acid-Vitamin C Serum for Skin Care.J Cosmet Dermatol. 2025 Jan;24(1):e16706. doi: 10.1111/jocd.16706. J Cosmet Dermatol. 2025. PMID: 39739360 Free PMC article.
-
Recent Advances in the Structural Design of Photosensitive Agent Formulations Using "Soft" Colloidal Nanocarriers.Pharmaceutics. 2020 Jun 24;12(6):587. doi: 10.3390/pharmaceutics12060587. Pharmaceutics. 2020. PMID: 32599791 Free PMC article. Review.
-
Supplementing Rhodobacter sphaeroides in the diet of lactating Holstein cows may naturally produce coenzyme Q10-enriched milk.Asian-Australas J Anim Sci. 2018 Jan;31(1):40-46. doi: 10.5713/ajas.17.0153. Epub 2017 Apr 21. Asian-Australas J Anim Sci. 2018. PMID: 28427254 Free PMC article.
-
Determination of coenzyme Q10 content in raw materials and dietary supplements by high-performance liquid chromatography-UV: collaborative study.J AOAC Int. 2008 Jul-Aug;91(4):702-8. J AOAC Int. 2008. PMID: 18727527 Free PMC article.
References
-
- Crane FL, Hatefi Y, Lester RI, Widmer C. Biochimica et Biophys. Acta. 1957;25:220–221. - PubMed
-
- Wolf DE, Hoffman CH, Trenner NR, Arison BH, Shunk CH, Linn BD, McPherson JF, Folkers K. J. Am. Chem. Soc. 1958;80:4752–4758.
-
- Alleva R, Scaraarmucci A, Mantera F, Bompandre S, Leoni L, Linarro GP. Mol. Aspect. Med. 1997;18:221–228.
-
- Raitakari OT, McCredie RJ, Witting P, Griffiths KA, Letter J, Sullivan D, Stocker R, Celermajer DS. Free Radic. Biol. Med. 2000;28:1100–1105. - PubMed
-
- Langsjoen P, Langsjoen A, Willis R, Folkers K. Mol. Aspect. Med. 1994;15:S265–S272. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
