gammaH2AX foci form preferentially in euchromatin after ionising-radiation
- PMID: 17957241
- PMCID: PMC2020439
- DOI: 10.1371/journal.pone.0001057
gammaH2AX foci form preferentially in euchromatin after ionising-radiation
Abstract
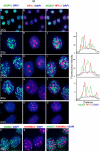
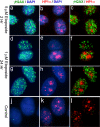
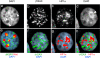
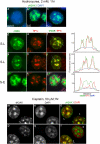
Background: The histone variant histone H2A.X comprises up to 25% of the H2A complement in mammalian cells. It is rapidly phosphorylated following exposure of cells to double-strand break (DSB) inducing agents such as ionising radiation. Within minutes of DSB generation, H2AX molecules are phosphorylated in large chromatin domains flanking DNA double-strand breaks (DSBs); these domains can be observed by immunofluorescence microscopy and are termed gammaH2AX foci. H2AX phosphorylation is believed to have a role mounting an efficient cellular response to DNA damage. Theoretical considerations suggest an essentially random chromosomal distribution of X-ray induced DSBs, and experimental evidence does not consistently indicate otherwise. However, we observed an apparently uneven distribution of gammaH2AX foci following X-irradiation with regions of the nucleus devoid of foci.
Methodology/principle findings: Using immunofluorescence microscopy, we show that focal phosphorylation of histone H2AX occurs preferentially in euchromatic regions of the genome following X-irradiation. H2AX phosphorylation has also been demonstrated previously to occur at stalled replication forks induced by UV radiation or exposure to agents such as hydroxyurea. In this study, treatment of S-phase cells with hydroxyurea lead to efficient H2AX phosphorylation in both euchromatin and heterochromatin at times when these chromatin compartments were undergoing replication. This suggests a block to H2AX phosphorylation in heterochromatin that is at least partially relieved by ongoing DNA replication.
Conclusions/significance: We discuss a number of possible mechanisms that could account for the observed pattern of H2AX phosphorylation. Since gammaH2AX is regarded as forming a platform for the recruitment or retention of other DNA repair and signaling molecules, these findings imply that the processing of DSBs in heterochromatin differs from that in euchromatic regions. The differential responses of heterochromatic and euchromatic compartments of the genome to DSBs will have implications for understanding the processes of DNA repair in relation to nuclear and chromatin organization.
Conflict of interest statement
Figures

References
-
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. - PubMed
-
- Redon C, Pilch D, Rogakou E, Sedelnikova O, Newrock K, et al. Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev. 2002;12:162–169. - PubMed
-
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. - PubMed
-
- Shiloh Y. The ATM-mediated DNA-damage response: taking shape. Trends Biochem Sci. 2006;31:402–410. - PubMed
-
- Stiff T, O'Driscoll M, Rief N, Iwabuchi K, Lobrich M, et al. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

