Protection of rhesus monkeys by a DNA prime/poxvirus boost malaria vaccine depends on optimal DNA priming and inclusion of blood stage antigens
- PMID: 17957247
- PMCID: PMC2031826
- DOI: 10.1371/journal.pone.0001063
Protection of rhesus monkeys by a DNA prime/poxvirus boost malaria vaccine depends on optimal DNA priming and inclusion of blood stage antigens
Abstract
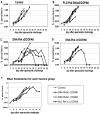
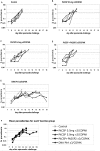
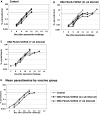
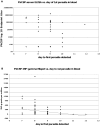
Background: We have previously described a four antigen malaria vaccine consisting of DNA plasmids boosted by recombinant poxviruses which protects a high percentage of rhesus monkeys against Plasmodium knowlesi (Pk) malaria. This is a multi-stage vaccine that includes two pre-erythrocytic antigens, PkCSP and PkSSP2(TRAP), and two erythrocytic antigens, PkAMA-1 and PkMSP-1(42kD). The present study reports three further experiments where we investigate the effects of DNA dose, timing, and formulation. We also compare vaccines utilizing only the pre-erythrocytic antigens with the four antigen vaccine.
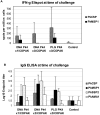
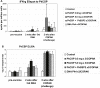
Methodology: In three experiments, rhesus monkeys were immunized with malaria vaccines using DNA plasmid injections followed by boosting with poxvirus vaccine. A variety of parameters were tested, including formulation of DNA on poly-lactic co-glycolide (PLG) particles, varying the number of DNA injections and the amount of DNA, varying the interval between the last DNA injection to the poxvirus boost from 7 to 21 weeks, and using vaccines with from one to four malaria antigens. Monkeys were challenged with Pk sporozoites given i.v. 2 to 4 weeks after the poxvirus injection, and parasitemia was measured by daily Giemsa stained blood films. Immune responses in venous blood samples taken after each vaccine injection were measured by ELIspot production of interferon-gamma, and by ELISA.
Conclusions: 1) the number of DNA injections, the formulation of the DNA plasmids, and the interval between the last DNA injection and the poxvirus injection are critical to vaccine efficacy. However, the total dose used for DNA priming is not as important; 2) the blood stage antigens PkAMA-1 and PkMSP-1 were able to protect against high parasitemias as part of a genetic vaccine where antigen folding is not well defined; 3) immunization with PkSSP2 DNA inhibited immune responses to PkCSP DNA even when vaccinations were given into separate legs; and 4) in a counter-intuitive result, higher interferon-gamma ELIspot responses to the PkCSP antigen correlated with earlier appearance of parasites in the blood, despite the fact that PkCSP vaccines had a protective effect.
Conflict of interest statement
Figures
References
-
- Ballou WR. Malaria vaccines in development. Expert Opin Emerg Drugs. 2005;10:489–503. - PubMed
-
- Ballou WR, Arevalo-Herrera M, Carucci D, Richie TL, Corradin G, et al. Update on the clinical development of candidate malaria vaccines. Am J Trop Med Hyg. 2004;71:239–247. - PubMed
-
- Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–1420. - PubMed
-
- Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366:2012–2018. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

