Metamorphosis of an identified serotonergic neuron in the Drosophila olfactory system
- PMID: 17958902
- PMCID: PMC2129096
- DOI: 10.1186/1749-8104-2-20
Metamorphosis of an identified serotonergic neuron in the Drosophila olfactory system
Abstract
Background: Odors are detected by sensory neurons that carry information to the olfactory lobe where they connect to projection neurons and local interneurons in glomeruli: anatomically well-characterized structures that collect, integrate and relay information to higher centers. Recent studies have revealed that the sensitivity of such networks can be modulated by wide-field feedback neurons. The connectivity and function of such feedback neurons are themselves subject to alteration by external cues, such as hormones, stress, or experience. Very little is known about how this class of central neurons changes its anatomical properties to perform functions in altered developmental contexts. A mechanistic understanding of how central neurons change their anatomy to meet new functional requirements will benefit greatly from the establishment of a model preparation where cellular and molecular changes can be examined in an identified central neuron.
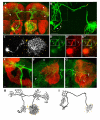
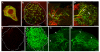
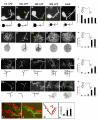
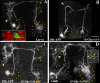
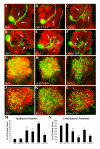
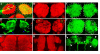
Results: In this study, we examine a wide-field serotonergic neuron in the Drosophila olfactory pathway and map the dramatic changes that it undergoes from larva to adult. We show that expression of a dominant-negative form of the ecdysterone receptor prevents remodeling. We further use different transgenic constructs to silence neuronal activity and report defects in the morphology of the adult-specific dendritic trees. The branching of the presynaptic axonal arbors is regulated by mechanisms that affect axon growth and retrograde transport. The neuron develops its normal morphology in the absence of sensory input to the antennal lobe, or of the mushroom bodies. However, ablation of its presumptive postsynaptic partners, the projection neurons and/or local interneurons, affects the growth and branching of terminal arbors.
Conclusion: Our studies establish a cellular system for studying remodeling of a central neuromodulatory feedback neuron and also identify key elements in this process. Understanding the morphogenesis of such neurons, which have been shown in other systems to modulate the sensitivity and directionality of response to odors, links anatomy to the development of olfactory behavior.
Figures

Similar articles
-
Developmentally programmed remodeling of the Drosophila olfactory circuit.Development. 2005 Feb;132(4):725-37. doi: 10.1242/dev.01614. Epub 2005 Jan 19. Development. 2005. PMID: 15659487
-
Spatial representation of the glomerular map in the Drosophila protocerebrum.Cell. 2002 Apr 19;109(2):229-41. doi: 10.1016/s0092-8674(02)00707-9. Cell. 2002. PMID: 12007409
-
Representation of the glomerular olfactory map in the Drosophila brain.Cell. 2002 Apr 19;109(2):243-55. doi: 10.1016/s0092-8674(02)00700-6. Cell. 2002. PMID: 12007410
-
Development of olfactory projection neuron dendrites that contribute to wiring specificity of the Drosophila olfactory circuit.Genes Genet Syst. 2014;89(1):17-26. doi: 10.1266/ggs.89.17. Genes Genet Syst. 2014. PMID: 24817758 Review.
-
How smell develops.Nat Neurosci. 2001 Nov;4 Suppl:1192-8. doi: 10.1038/nn751. Nat Neurosci. 2001. PMID: 11687829 Review.
Cited by
-
Neuronal polarity in Drosophila: sorting out axons and dendrites.Dev Neurobiol. 2011 Jun;71(6):419-29. doi: 10.1002/dneu.20836. Dev Neurobiol. 2011. PMID: 21557498 Free PMC article. Review.
-
Identified Serotonin-Releasing Neurons Induce Behavioral Quiescence and Suppress Mating in Drosophila.J Neurosci. 2015 Sep 16;35(37):12792-812. doi: 10.1523/JNEUROSCI.1638-15.2015. J Neurosci. 2015. PMID: 26377467 Free PMC article.
-
The anatomical basis for modulatory convergence in the antennal lobe of Manduca sexta.J Comp Neurol. 2016 Jun 15;524(9):1859-75. doi: 10.1002/cne.23926. Epub 2015 Dec 29. J Comp Neurol. 2016. PMID: 26560074 Free PMC article.
-
Transcriptional changes in specific subsets of Drosophila neurons following inhibition of the serotonin transporter.Res Sq [Preprint]. 2023 Mar 17:rs.3.rs-2626506. doi: 10.21203/rs.3.rs-2626506/v1. Res Sq. 2023. Update in: Transl Psychiatry. 2023 Jun 24;13(1):226. doi: 10.1038/s41398-023-02521-3. PMID: 36993644 Free PMC article. Updated. Preprint.
-
The role of astrocyte-mediated plasticity in neural circuit development and function.Neural Dev. 2021 Jan 7;16(1):1. doi: 10.1186/s13064-020-00151-9. Neural Dev. 2021. PMID: 33413602 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

