Drosophila glypican Dally-like acts in FGF-receiving cells to modulate FGF signaling during tracheal morphogenesis
- PMID: 17959166
- PMCID: PMC2151973
- DOI: 10.1016/j.ydbio.2007.09.015
Drosophila glypican Dally-like acts in FGF-receiving cells to modulate FGF signaling during tracheal morphogenesis
Abstract
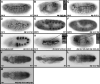
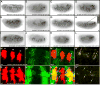
Previous studies in Drosophila have shown that heparan sulfate proteoglycans (HSPGs) are involved in both breathless (btl)- and heartless (htl)-mediated FGF signaling during embryogenesis. However, the mechanism(s) by which HSPGs control Btl and Htl signaling is unknown. Here we show that dally-like (dlp, a Drosophila glypican) mutant embryos exhibit severe defects in tracheal morphogenesis and show a reduction in btl-mediated FGF signaling activity. However, htl-dependent mesodermal cell migration is not affected in dlp mutant embryos. Furthermore, expression of Dlp, but not other Drosophila HSPGs, can restore effectively the tracheal morphogenesis in dlp embryos. Rescue experiments in dlp embryos demonstrate that Dlp functions only in Bnl/FGF receiving cells in a cell-autonomous manner, but is not essential for Bnl/FGF expression cells. To further dissect the mechanism(s) of Dlp in Btl signaling, we analyzed the role of Dlp in Btl-mediated air sac tracheoblast formation in wing discs. Mosaic analysis experiments show that removal of HSPG activity in FGF-producing or other surrounding cells does not affect tracheoblasts migration, while HSPG mutant tracheoblast cells fail to receive FGF signaling. Together, our results argue strongly that HSPGs regulate Btl signaling exclusively in FGF-receiving cells as co-receptors, but are not essential for the secretion and distribution of the FGF ligand. This mechanism is distinct from HSPG functions in morphogen distribution, and is likely a general paradigm for HSPG functions in FGF signaling in Drosophila.
Figures



Similar articles
-
Unique patterns of organization and migration of FGF-expressing cells during Drosophila morphogenesis.Dev Biol. 2017 Jul 1;427(1):35-48. doi: 10.1016/j.ydbio.2017.05.009. Epub 2017 May 11. Dev Biol. 2017. PMID: 28502613 Free PMC article.
-
Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc.Development. 2005 Feb;132(4):667-79. doi: 10.1242/dev.01636. Epub 2005 Jan 12. Development. 2005. PMID: 15647319
-
Cells must express components of the planar cell polarity system and extracellular matrix to support cytonemes.Elife. 2016 Sep 3;5:e18979. doi: 10.7554/eLife.18979. Elife. 2016. PMID: 27591355 Free PMC article.
-
Dual roles of Drosophila glypican Dally-like in Wingless/Wnt signaling and distribution.Methods Enzymol. 2010;480:33-50. doi: 10.1016/S0076-6879(10)80002-3. Methods Enzymol. 2010. PMID: 20816203 Review.
-
The role of FGF signaling in guiding coordinate movement of cell groups: guidance cue and cell adhesion regulator?Cell Adh Migr. 2012 Sep-Oct;6(5):397-403. doi: 10.4161/cam.21103. Epub 2012 Sep 1. Cell Adh Migr. 2012. PMID: 23076054 Free PMC article. Review.
Cited by
-
The Air Sac Primordium of Drosophila: A Model for Invasive Development.Int J Mol Sci. 2018 Jul 17;19(7):2074. doi: 10.3390/ijms19072074. Int J Mol Sci. 2018. PMID: 30018198 Free PMC article. Review.
-
Structure of the protein core of the glypican Dally-like and localization of a region important for hedgehog signaling.Proc Natl Acad Sci U S A. 2011 Aug 9;108(32):13112-7. doi: 10.1073/pnas.1109877108. Epub 2011 Jul 26. Proc Natl Acad Sci U S A. 2011. PMID: 21828006 Free PMC article.
-
Glycosaminoglycan-dependent restriction of FGF diffusion is necessary for lacrimal gland development.Development. 2012 Aug;139(15):2730-9. doi: 10.1242/dev.079236. Epub 2012 Jun 28. Development. 2012. PMID: 22745308 Free PMC article.
-
Glypicans.Genome Biol. 2008;9(5):224. doi: 10.1186/gb-2008-9-5-224. Epub 2008 May 22. Genome Biol. 2008. PMID: 18505598 Free PMC article. Review.
-
Glypican-1 regulates myoblast response to HGF via Met in a lipid raft-dependent mechanism: effect on migration of skeletal muscle precursor cells.Skelet Muscle. 2014 Feb 12;4(1):5. doi: 10.1186/2044-5040-4-5. Skelet Muscle. 2014. PMID: 24517345 Free PMC article.
References
-
- Affolter M, Weijer CJ. Signaling to cytoskeletal dynamics during chemotaxis. Dev Cell. 2005;9:19–34. - PubMed
-
- Baeg GH, et al. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. - PubMed
-
- Baeg GH, et al. The Wingless morphogen gradient is established by the cooperative action of Frizzled and Heparan Sulfate Proteoglycan receptors. Dev Biol. 2004;276:89–100. - PubMed
-
- Beiman M, et al. Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev. 1996;10:2993–3002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

