Pathogenic role of B cells and antibodies in murine Leishmania amazonensis infection
- PMID: 17959178
- PMCID: PMC2276857
- DOI: 10.1016/j.ijpara.2007.08.010
Pathogenic role of B cells and antibodies in murine Leishmania amazonensis infection
Abstract
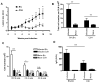
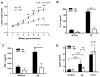
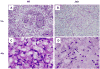
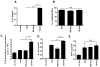
Leishmania amazonensis infection, occurring predominantly in Central and South America, can manifest itself in several forms, including those of cutaneous and diffuse cutaneous leishmaniasis. The outcome of L. amazonensis infection depends largely on host immune responses to the parasites. While CD4+ T cell activation is a prerequisite for pathogenesis in L. amazonensis-infected mice, the roles of B cells and their antibody production are unclear. In this study, we provide evidence suggesting that B cells and antibodies are involved in disease pathogenesis. We documented a correlation between B cell activation and lesion progress in immunocompetent mice. In the absence of functional B cells and antibodies, JhD mice showed a delayed onset of disease and developed small lesions. Histological examination of these mice revealed a significant reduction in CD4+ and CD8+ T cells, but not in MAC1+ macrophages, at the infection site. In contrast to the wild-type mice that showed typical tissue necrosis, L. amazonensis-infected JhD mice showed no or minimal signs of necrotic foci. A marked reduction in CD4+ T cell proliferation and cytokine (IFN-gamma and IL-10) production in infected JhD mice suggested an involvement of B cells and antibodies in the priming of parasite-specific T cells. This notion was further supported by the observations that adoptive transfer of B cells or antibodies could restore CD4+ T cell activation and migration in infected JhD mice. Moreover, antibody coating of parasites could stimulate dendritic cells to produce high levels of cytokines and increase their ability to prime nai ve CD4+ T cells. Since CD4+ T cells are crucial to disease pathogenesis, this study suggests that B cells and their antibody production enhanced L. amazonensis infection, partially by promoting T cell priming and cellular migration to the infection site.
Figures


Similar articles
-
Leishmania pifanoi pathogenesis: selective lack of a local cutaneous response in the absence of circulating antibody.Infect Immun. 2002 Dec;70(12):6597-605. doi: 10.1128/IAI.70.12.6597-6605.2002. Infect Immun. 2002. PMID: 12438331 Free PMC article.
-
IFN-γ-Dependent Recruitment of CD4(+) T Cells and Macrophages Contributes to Pathogenesis During Leishmania amazonensis Infection.J Interferon Cytokine Res. 2015 Dec;35(12):935-47. doi: 10.1089/jir.2015.0043. Epub 2015 Sep 24. J Interferon Cytokine Res. 2015. PMID: 26401717 Free PMC article.
-
Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4(+) T cells in vivo.J Immunol. 2001 Oct 15;167(8):4534-42. doi: 10.4049/jimmunol.167.8.4534. J Immunol. 2001. PMID: 11591781
-
Reviewing the role of the dendritic Langerhans cells in the immunopathogenesis of American cutaneous leishmaniasis.Trans R Soc Trop Med Hyg. 2008 Nov;102(11):1075-80. doi: 10.1016/j.trstmh.2008.05.020. Epub 2008 Jul 3. Trans R Soc Trop Med Hyg. 2008. PMID: 18602127 Review.
-
The Paradox of a Phagosomal Lifestyle: How Innate Host Cell-Leishmania amazonensis Interactions Lead to a Progressive Chronic Disease.Front Immunol. 2021 Sep 7;12:728848. doi: 10.3389/fimmu.2021.728848. eCollection 2021. Front Immunol. 2021. PMID: 34557194 Free PMC article. Review.
Cited by
-
The role of TLR9 on Leishmania amazonensis infection and its influence on intranasal LaAg vaccine efficacy.PLoS Negl Trop Dis. 2019 Feb 25;13(2):e0007146. doi: 10.1371/journal.pntd.0007146. eCollection 2019 Feb. PLoS Negl Trop Dis. 2019. PMID: 30802247 Free PMC article.
-
What do we know about the role of regulatory B cells (Breg) during the course of infection of two major parasitic diseases, malaria and leishmaniasis?Pathog Glob Health. 2017 May;111(3):107-115. doi: 10.1080/20477724.2017.1308902. Epub 2017 Mar 29. Pathog Glob Health. 2017. PMID: 28353409 Free PMC article. Review.
-
IL-4-producing B cells regulate T helper cell dichotomy in type 1- and type 2-controlled diseases.Proc Natl Acad Sci U S A. 2017 Oct 3;114(40):E8430-E8439. doi: 10.1073/pnas.1708125114. Epub 2017 Sep 15. Proc Natl Acad Sci U S A. 2017. PMID: 28916732 Free PMC article.
-
Subversion of Immunity by Leishmania amazonensis Parasites: Possible Role of Phosphatidylserine as a Main Regulator.J Parasitol Res. 2012;2012:981686. doi: 10.1155/2012/981686. Epub 2012 Feb 2. J Parasitol Res. 2012. PMID: 22518276 Free PMC article.
-
An in vitro model of antibody-enhanced killing of the intracellular parasite Leishmania amazonensis.PLoS One. 2014 Sep 5;9(9):e106426. doi: 10.1371/journal.pone.0106426. eCollection 2014. PLoS One. 2014. PMID: 25191842 Free PMC article.
References
-
- Anderson CF, Gerber JS, Mosser DM. Modulating macrophage function with IgG immune complexes. J Endotoxin Res. 2002;8:477–81. - PubMed
-
- Barnes N, Gavin AL, Tan PS, Mottram P, Koentgen F, Hogarth PM. FcgammaRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity. 2002;16:379–89. - PubMed
-
- Constant SL. B lymphocytes as antigen-presenting cells for CD4+ T cell priming in vivo. J Immunol. 1999;162:5695–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

