Peptide-conjugated morpholino oligomers inhibit porcine reproductive and respiratory syndrome virus replication
- PMID: 17959259
- PMCID: PMC7114306
- DOI: 10.1016/j.antiviral.2007.09.002
Peptide-conjugated morpholino oligomers inhibit porcine reproductive and respiratory syndrome virus replication
Abstract
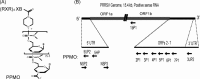
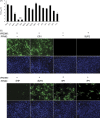
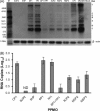
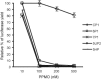
Porcine reproductive and respiratory syndrome (PRRS) has been devastating the global swine industry for more than a decade, and current strategies to control PRRS are inadequate. In this study we characterized the inhibition of PRRS virus (PRRSV) replication by antisense phosphorodiamidate morpholino oligomers (PMO). Of 12 peptide-conjugated PMO (PPMO), four were found to be highly effective at inhibiting PRRSV replication in cell culture in a dose-dependant and sequence-specific manner. PPMO 5UP2 and 5HP are complementary to sequence in the 5' end of the PRRSV genome, and 6P1 and 7P1 to sequence in the translation initiation regions of ORF6 and ORF7, respectively. Treatment of cells with 5UP2 or 5HP caused a 4.5log(10) reduction in PRRSV yield, compared to a control PPMO. Combination of 6P1 and 7P1 led to higher level reduction than 6P1 or 7P1 alone. 5UP2, 5HP, and a combination of 6P1 and 7P1 inhibited PRRSV replication in porcine alveolar macrophages and protected the cells from PRRSV-induced cytopathic effect. Northern blot and real-time RT-PCR results demonstrated that the effective PPMO led to a reduction of PRRSV RNA level. 5UP2 and 5HP inhibited virus replication of 10 other strains of PRRSV. Results from this study suggest potential applications of PPMO for PRRS control.
Figures

Similar articles
-
Inhibition of porcine reproductive and respiratory syndrome virus infection in piglets by a peptide-conjugated morpholino oligomer.Antiviral Res. 2011 Jul;91(1):36-42. doi: 10.1016/j.antiviral.2011.04.012. Epub 2011 Apr 30. Antiviral Res. 2011. PMID: 21554902
-
Morpholino oligomer-mediated protection of porcine pulmonary alveolar macrophages from arterivirus-induced cell death.Antivir Ther. 2009;14(7):899-909. doi: 10.3851/IMP1409. Antivir Ther. 2009. PMID: 19918094
-
Enhanced inhibition of porcine reproductive and respiratory syndrome virus replication by combination of morpholino oligomers.Antiviral Res. 2009 Apr;82(1):59-66. doi: 10.1016/j.antiviral.2009.01.009. Epub 2009 Feb 10. Antiviral Res. 2009. PMID: 19428596 Free PMC article.
-
Inhibition of RNA virus infections with peptide-conjugated morpholino oligomers.Curr Pharm Des. 2008;14(25):2619-34. doi: 10.2174/138161208786071290. Curr Pharm Des. 2008. PMID: 18991679 Review.
-
Antiviral Strategies against PRRSV Infection.Trends Microbiol. 2017 Dec;25(12):968-979. doi: 10.1016/j.tim.2017.06.001. Epub 2017 Jun 23. Trends Microbiol. 2017. PMID: 28652073 Review.
Cited by
-
PRRSV Vaccine Strain-Induced Secretion of Extracellular ISG15 Stimulates Porcine Alveolar Macrophage Antiviral Response against PRRSV.Viruses. 2020 Sep 10;12(9):1009. doi: 10.3390/v12091009. Viruses. 2020. PMID: 32927637 Free PMC article.
-
Proteomic Analysis of ISGylation in Immortalized Porcine Alveolar Macrophage Cell Lines Induced by Type I Interferon.Vaccines (Basel). 2021 Feb 17;9(2):164. doi: 10.3390/vaccines9020164. Vaccines (Basel). 2021. PMID: 33671165 Free PMC article.
-
Porcine reproductive and respiratory syndrome virus inhibits type I interferon signaling by blocking STAT1/STAT2 nuclear translocation.J Virol. 2010 Nov;84(21):11045-55. doi: 10.1128/JVI.00655-10. Epub 2010 Aug 25. J Virol. 2010. PMID: 20739522 Free PMC article.
-
Direct Interaction Between CD163 N-Terminal Domain and MYH9 C-Terminal Domain Contributes to Porcine Reproductive and Respiratory Syndrome Virus Internalization by Permissive Cells.Front Microbiol. 2019 Aug 6;10:1815. doi: 10.3389/fmicb.2019.01815. eCollection 2019. Front Microbiol. 2019. PMID: 31447818 Free PMC article.
-
Lipid rafts both in cellular membrane and viral envelope are critical for PRRSV efficient infection.Virology. 2015 Oct;484:170-180. doi: 10.1016/j.virol.2015.06.005. Epub 2015 Jun 23. Virology. 2015. PMID: 26115164 Free PMC article.
References
-
- Abes S., Moulton H.M., Clair P., Prevot P., Youngblood D.S., Wu R.P., Iversen P.L., Lebleu B. Vectorization of morpholino oligomers by the (R-Ahx-R)4 peptide allows efficient splicing correction in the absence of endosomolytic agents. J. Control. Release. 2006;116:304–313. - PubMed
-
- Arora V., Cate M.L., Ghosh C., Iversen P.L. Phosphorodiamidate morpholino antisense oligomers inhibit expression of human cytochrome P450 3A4 and alter selected drug metabolism. Drug Metab. Dispos. 2002;30:757–762. - PubMed
-
- Arora V., Knapp D.C., Reddy M.T., Weller D.D., Iversen P.L. Bioavailability and efficacy of antisense morpholino oligomers targeted to c-myc and cytochrome P-450 3A2 following oral administration in rats. J. Pharm. Sci. 2002;91:1009–1018. - PubMed
-
- Bautista E.M., Goyal S.M., Yoon I.J., Joo H.S., Collins J.E. Comparison of porcine alveolar macrophages and CL 2621 for the detection of porcine reproductive and respiratory syndrome (PRRS) virus and anti-PRRS antibody. J. Vet. Diagn. Invest. 1993;5:163–165. - PubMed
-
- Benfield D.A., Nelson E., Collins J.E., Harris L., Goyal S.M., Bobinson D., Christianson W.T., Morrison R.B., Gorcyca D., Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332) J. Vet. Diagn. Invest. 1992;4:127–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

