Human astrovirus coat protein inhibits serum complement activation via C1, the first component of the classical pathway
- PMID: 17959658
- PMCID: PMC2224607
- DOI: 10.1128/JVI.01847-07
Human astrovirus coat protein inhibits serum complement activation via C1, the first component of the classical pathway
Abstract
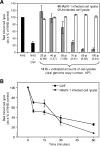
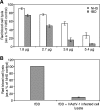
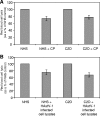
Human astroviruses (HAstVs) belong to a family of nonenveloped, icosahedral RNA viruses that cause noninflammatory gastroenteritis, predominantly in infants. Eight HAstV serotypes have been identified, with a worldwide distribution. While the HAstVs represent a significant public health concern, very little is known about the pathogenesis of and host immune response to these viruses. Here we demonstrate that HAstV type 1 (HAstV-1) virions, specifically the viral coat protein (CP), suppress the complement system, a fundamental component of the innate immune response in vertebrates. HAstV-1 virions and purified CP both suppress hemolytic complement activity. Hemolytic assays utilizing sera depleted of individual complement factors as well as adding back purified factors demonstrated that HAstV CP suppresses classical pathway activation at the first component, C1. HAstV-1 CP bound the A chain of C1q and inhibited serum complement activation, resulting in decreased C4b, iC3b, and terminal C5b-9 formation. Inhibition of complement activation was also demonstrated for HAstV serotypes 2 to 4, suggesting that this phenomenon is a general feature of these human pathogens. Since complement is a major contributor to the initiation and amplification of inflammation, the observed CP-mediated inhibition of complement activity may contribute to the lack of inflammation associated with astrovirus-induced gastroenteritis. Although diverse mechanisms of inhibition of complement activation have been described for many enveloped animal viruses, this is the first report of a nonenveloped icosahedral virus CP inhibiting classical pathway activation at C1.
Figures



Similar articles
-
Human astrovirus coat protein binds C1q and MBL and inhibits the classical and lectin pathways of complement activation.Mol Immunol. 2010 Jan;47(4):792-8. doi: 10.1016/j.molimm.2009.10.006. Epub 2009 Nov 6. Mol Immunol. 2010. PMID: 19896716
-
Potent inhibition of the classical pathway of complement by a novel C1q-binding peptide derived from the human astrovirus coat protein.Mol Immunol. 2010 Nov-Dec;48(1-3):305-13. doi: 10.1016/j.molimm.2010.07.012. Epub 2010 Aug 21. Mol Immunol. 2010. PMID: 20728940
-
Human astrovirus coat protein: a novel C1 inhibitor.Adv Exp Med Biol. 2008;632:237-51. Adv Exp Med Biol. 2008. PMID: 19025126 Review.
-
Structural, Mechanistic, and Antigenic Characterization of the Human Astrovirus Capsid.J Virol. 2015 Dec 9;90(5):2254-63. doi: 10.1128/JVI.02666-15. J Virol. 2015. PMID: 26656707 Free PMC article.
-
Molecular epidemiology of human astroviruses.Novartis Found Symp. 2001;238:237-45; discussion 245-9. doi: 10.1002/0470846534.ch14. Novartis Found Symp. 2001. PMID: 11444029 Review.
Cited by
-
Humoral innate immune response and disease.Clin Immunol. 2012 Aug;144(2):142-58. doi: 10.1016/j.clim.2012.06.002. Epub 2012 Jun 18. Clin Immunol. 2012. PMID: 22771788 Free PMC article. Review.
-
Viral-derived complement inhibitors: current status and potential role in immunomodulation.Exp Biol Med (Maywood). 2017 Feb;242(4):397-410. doi: 10.1177/1535370216675772. Epub 2016 Oct 26. Exp Biol Med (Maywood). 2017. PMID: 27798122 Free PMC article. Review.
-
Immunogenicity and Efficacy Evaluation of Subunit Astrovirus Vaccines.Vaccines (Basel). 2019 Aug 2;7(3):79. doi: 10.3390/vaccines7030079. Vaccines (Basel). 2019. PMID: 31382451 Free PMC article.
-
Inhibition of Immune Complex Complement Activation and Neutrophil Extracellular Trap Formation by Peptide Inhibitor of Complement C1.Front Immunol. 2018 Mar 26;9:558. doi: 10.3389/fimmu.2018.00558. eCollection 2018. Front Immunol. 2018. PMID: 29632531 Free PMC article.
-
Replication of Porcine Astrovirus Type 1-Infected PK-15 Cells In Vitro Affected by RIG-I and MDA5 Signaling Pathways.Microbiol Spectr. 2023 Jun 15;11(3):e0070123. doi: 10.1128/spectrum.00701-23. Epub 2023 May 4. Microbiol Spectr. 2023. PMID: 37140381 Free PMC article.
References
-
- Chung, K. M., M. K. Liszewski, G. Nybakken, A. E. Davis, R. R. Townsend, D. H. Fremont, J. P. Atkinson, and M. S. Diamond. 2006. West Nile virus nonstructural protein NS1 inhibits complement activation by binding the regulatory protein factor H. Proc. Natl. Acad. Sci. USA 10319111-19116. - PMC - PubMed
-
- Cooper, N. R. 1985. The classical complement pathway: activation and regulation of the first complement component. Adv. Immunol. 37151-216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

