Immunization with a lentivector that targets tumor antigen expression to dendritic cells induces potent CD8+ and CD4+ T-cell responses
- PMID: 17959670
- PMCID: PMC2224357
- DOI: 10.1128/JVI.01289-07
Immunization with a lentivector that targets tumor antigen expression to dendritic cells induces potent CD8+ and CD4+ T-cell responses
Abstract
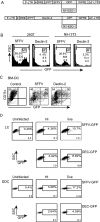
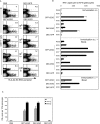
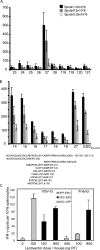
Lentivectors stimulate potent immune responses to antigen transgenes and are being developed as novel genetic vaccines. To improve safety while retaining efficacy, we constructed a lentivector in which transgene expression was restricted to antigen-presenting cells using the mouse dectin-2 gene promoter. This lentivector expressed a green fluorescent protein (GFP) transgene in mouse bone marrow-derived dendritic cell cultures and in human skin-derived Langerhans and dermal dendritic cells. In mice GFP expression was detected in splenic dectin-2(+) cells after intravenous injection and in CD11c(+) dendritic cells in the draining lymph node after subcutaneous injection. A dectin-2 lentivector encoding the human melanoma antigen NY-ESO-1 primed an NY-ESO-1-specific CD8(+) T-cell response in HLA-A2 transgenic mice and stimulated a CD4(+) T-cell response to a newly identified NY-ESO-1 epitope presented by H2 I-A(b). As immunization with the optimal dose of the dectin-2 lentivector was similar to that stimulated by a lentivector containing a strong constitutive viral promoter, targeting antigen expression to dendritic cells can provide a safe and effective vaccine.
Figures


Similar articles
-
Lentivector immunization induces tumor antigen-specific B and T cell responses in vivo.Eur J Immunol. 2008 Jul;38(7):1867-76. doi: 10.1002/eji.200737923. Eur J Immunol. 2008. PMID: 18546142
-
Lentivector immunization stimulates potent CD8 T cell responses against melanoma self-antigen tyrosinase-related protein 1 and generates antitumor immunity in mice.J Immunol. 2009 May 15;182(10):5960-9. doi: 10.4049/jimmunol.0900008. J Immunol. 2009. PMID: 19414747 Free PMC article.
-
Antibody-targeted NY-ESO-1 to mannose receptor or DEC-205 in vitro elicits dual human CD8+ and CD4+ T cell responses with broad antigen specificity.J Immunol. 2011 Jan 15;186(2):1218-27. doi: 10.4049/jimmunol.1000808. Epub 2010 Dec 13. J Immunol. 2011. PMID: 21149605
-
Lentivirus as a potent and mechanistically distinct vector for genetic immunization.Curr Opin Mol Ther. 2007 Oct;9(5):439-46. Curr Opin Mol Ther. 2007. PMID: 17932808 Free PMC article. Review.
-
Recombinant lentivector as a genetic immunization vehicle for antitumor immunity.Expert Rev Vaccines. 2007 Dec;6(6):913-24. doi: 10.1586/14760584.6.6.913. Expert Rev Vaccines. 2007. PMID: 18377355 Free PMC article. Review.
Cited by
-
Pros and Cons of Antigen-Presenting Cell Targeted Tumor Vaccines.J Immunol Res. 2015;2015:785634. doi: 10.1155/2015/785634. Epub 2015 Oct 25. J Immunol Res. 2015. PMID: 26583156 Free PMC article. Review.
-
Nonintegrating lentiviral vectors can effectively deliver ovalbumin antigen for induction of antitumor immunity.Hum Gene Ther. 2009 Dec;20(12):1652-64. doi: 10.1089/hum.2009.012. Hum Gene Ther. 2009. PMID: 19663564 Free PMC article.
-
Lentiviral vectors in gene therapy: their current status and future potential.Arch Immunol Ther Exp (Warsz). 2010 Apr;58(2):107-19. doi: 10.1007/s00005-010-0063-4. Epub 2010 Feb 9. Arch Immunol Ther Exp (Warsz). 2010. PMID: 20143172 Free PMC article. Review.
-
Dendritic cell-specific antigen delivery by coronavirus vaccine vectors induces long-lasting protective antiviral and antitumor immunity.mBio. 2010 Sep 14;1(4):e00171-10. doi: 10.1128/mBio.00171-10. mBio. 2010. PMID: 20844609 Free PMC article.
-
Production of lentiviral vectors with enhanced efficiency to target dendritic cells by attenuating mannosidase activity of mammalian cells.J Biol Eng. 2011 Jan 28;5(1):1. doi: 10.1186/1754-1611-5-1. J Biol Eng. 2011. PMID: 21276219 Free PMC article.
References
-
- Allan, R. S., C. M. Smith, G. T. Belz, A. L. van Lint, L. M. Wakim, W. R. Heath, and F. R. Carbone. 2003. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science 3011925-1928. - PubMed
-
- Allan, R. S., J. Waithman, S. Bedoui, C. M. Jones, J. A. Villadangos, Y. Zhan, A. M. Lew, K. Shortman, W. R. Heath, and F. R. Carbone. 2006. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25153-162. - PubMed
-
- Aragane, Y., A. Maeda, A. Schwarz, T. Tezuka, K. Ariizumi, and T. Schwarz. 2003. Involvement of dectin-2 in ultraviolet radiation-induced tolerance. J. Immunol. 1713801-3807. - PubMed
-
- Ariizumi, K., G. L. Shen, S. Shikano, R. Ritter, 3rd, P. Zukas, D. Edelbaum, A. Morita, and A. Takashima. 2000. Cloning of a second dendritic cell-associated C-type lectin (dectin-2) and its alternatively spliced isoforms. J. Biol. Chem. 27511957-11963. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

