Defects in embryonic neurogenesis and initial synapse formation in the forebrain of the Ts65Dn mouse model of Down syndrome
- PMID: 17959791
- PMCID: PMC6673208
- DOI: 10.1523/JNEUROSCI.3406-07.2007
Defects in embryonic neurogenesis and initial synapse formation in the forebrain of the Ts65Dn mouse model of Down syndrome
Abstract
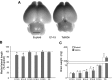
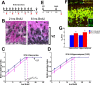
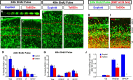
Trisomy 21, one of the most prevalent congenital birth defects, results in a constellation of phenotypes collectively termed Down syndrome (DS). Mental retardation and motor and sensory deficits are among the many debilitating symptoms of DS. Alterations in brain growth and synaptic development are thought to underlie the cognitive impairments in DS, but the role of early brain development has not been studied because of the lack of embryonic human tissue and because of breeding difficulties in mouse models of DS. We generated a breeding colony of the Ts65Dn mouse model of DS to test the hypothesis that early defects in embryonic brain development are a component of brain dysfunction in DS. We found substantial delays in prenatal growth of the Ts65Dn cerebral cortex and hippocampus because of longer cell cycle duration and reduced neurogenesis from the ventricular zone neural precursor population. In addition, the Ts65Dn neocortex remains hypocellular after birth and there is a lasting decrease in synaptic development beginning in the first postnatal week. These results demonstrate that specific abnormalities in embryonic forebrain precursor cells precede early deficits in synaptogenesis and may underlie the postnatal disabilities in Ts65Dn and DS. The early prenatal period is therefore an important new window for possible therapeutic amelioration of the cognitive symptoms in DS.
Figures




Similar articles
-
Lifespan analysis of brain development, gene expression and behavioral phenotypes in the Ts1Cje, Ts65Dn and Dp(16)1/Yey mouse models of Down syndrome.Dis Model Mech. 2018 Jun 12;11(6):dmm031013. doi: 10.1242/dmm.031013. Dis Model Mech. 2018. PMID: 29716957 Free PMC article.
-
Maternal choline supplementation improves spatial learning and adult hippocampal neurogenesis in the Ts65Dn mouse model of Down syndrome.Neurobiol Dis. 2013 Oct;58:92-101. doi: 10.1016/j.nbd.2013.04.016. Epub 2013 Apr 30. Neurobiol Dis. 2013. PMID: 23643842 Free PMC article.
-
Short- and long-term effects of neonatal pharmacotherapy with epigallocatechin-3-gallate on hippocampal development in the Ts65Dn mouse model of Down syndrome.Neuroscience. 2016 Oct 1;333:277-301. doi: 10.1016/j.neuroscience.2016.07.031. Epub 2016 Jul 25. Neuroscience. 2016. PMID: 27457036
-
On the cause of mental retardation in Down syndrome: extrapolation from full and segmental trisomy 16 mouse models.Brain Res Brain Res Rev. 2001 Apr;35(2):115-45. doi: 10.1016/s0926-6410(00)00074-4. Brain Res Brain Res Rev. 2001. PMID: 11336779 Review.
-
Alterations of brain circuits in Down syndrome murine models.J Chem Neuroanat. 2011 Dec;42(4):317-26. doi: 10.1016/j.jchemneu.2011.09.002. Epub 2011 Sep 16. J Chem Neuroanat. 2011. PMID: 21946025 Review.
Cited by
-
Commonality in Down and fetal alcohol syndromes.Birth Defects Res A Clin Mol Teratol. 2013 Apr;97(4):187-97. doi: 10.1002/bdra.23129. Epub 2013 Apr 3. Birth Defects Res A Clin Mol Teratol. 2013. PMID: 23554291 Free PMC article.
-
An Integrated Human/Murine Transcriptome and Pathway Approach To Identify Prenatal Treatments For Down Syndrome.Sci Rep. 2016 Sep 2;6:32353. doi: 10.1038/srep32353. Sci Rep. 2016. PMID: 27586445 Free PMC article.
-
Altered synaptic marker abundance in the hippocampal stratum oriens of Ts65Dn mice is associated with exuberant expression of versican.ASN Neuro. 2012 Feb 8;4(1):e00073. doi: 10.1042/AN20110037. ASN Neuro. 2012. PMID: 22225533 Free PMC article.
-
Neurogenesis and neuronal differentiation in the postnatal frontal cortex in Down syndrome.Acta Neuropathol Commun. 2022 Jun 8;10(1):86. doi: 10.1186/s40478-022-01385-w. Acta Neuropathol Commun. 2022. PMID: 35676735 Free PMC article.
-
Developmental and adult GAP-43 deficiency in mice dynamically alters hippocampal neurogenesis and mossy fiber volume.Dev Neurosci. 2014;36(1):44-63. doi: 10.1159/000357840. Epub 2014 Feb 26. Dev Neurosci. 2014. PMID: 24576816 Free PMC article.
References
-
- Altman J, Bayer SA. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J Comp Neurol. 1990;301:325–342. - PubMed
-
- Baxter LL, Moran TH, Richtsmeier JT, Troncoso J, Reeves RH. Discovery and genetic localization of Down syndrome cerebellar phenotypes using the Ts65Dn mouse. Hum Mol Genet. 2000;9:195–202. - PubMed
-
- Becker LE, Armstrong DL, Chan F. Dendritic atrophy in children with Down's syndrome. Ann Neurol. 1986;20:520–526. - PubMed
-
- Belichenko PV, Masliah E, Kleschevnikov AM, Villar AJ, Epstein CJ, Salehi A, Mobley WC. Synaptic structural abnormalities in the Ts65Dn mouse model of down syndrome. J Comp Neurol. 2004;480:281–298. - PubMed
-
- Benavides-Piccione R, Ballesteros-Yanez I, de Lagran MM, Elston G, Estivill X, Fillat C, Defelipe J, Dierssen M. On dendrites in Down syndrome and DS murine models: a spiny way to learn. Prog Neurobiol. 2004;74:111–126. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
