Regulation of Na(v)1.2 channels by brain-derived neurotrophic factor, TrkB, and associated Fyn kinase
- PMID: 17959796
- PMCID: PMC6673213
- DOI: 10.1523/JNEUROSCI.5005-06.2007
Regulation of Na(v)1.2 channels by brain-derived neurotrophic factor, TrkB, and associated Fyn kinase
Abstract
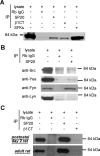
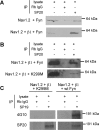
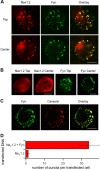
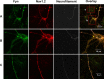
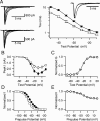
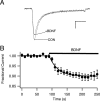
Voltage-gated sodium channels are responsible for action potential initiation and propagation in neurons, and modulation of their function has an important impact on neuronal excitability. Sodium channels are regulated by a Src-family tyrosine kinase pathway, and this modulation can be reversed by specifically bound receptor phosphoprotein tyrosine phosphatase-beta. However, the specific tyrosine kinase and signaling pathway are unknown. We found that the sodium channels in rat brain interact with Fyn, one of four Src-family tyrosine kinases expressed in the brain. Na(V)1.2 channels and Fyn are localized together in the axons of cultured hippocampal neurons, the mossy fibers of the hippocampus, and cell bodies, dendrites, and axons of neurons in many other brain areas, and they coimmunoprecipitate with Fyn from cotransfected tsA-201 cells. Coexpression of Fyn with Na(V)1.2 channels decreases sodium currents by increasing the rate of inactivation and causing a negative shift in the voltage dependence of inactivation. Reconstitution of a signaling pathway from brain-derived neurotrophic factor (BDNF) to sodium channels via the tyrosine receptor kinase B (TrkB)/p75 neurotrophin receptor and Fyn kinase in transfected cells resulted in an increased rate of inactivation of sodium channels and a negative shift in the voltage dependence of inactivation after treatment with BDNF. These results indicate that Fyn kinase is associated with sodium channels in brain neurons and can modulate Na(V)1.2 channels by tyrosine phosphorylation after activation of TrkB/p75 signaling by BDNF.
Figures


References
-
- Baranauskas G, Nistri A. Sensitization of pain pathways in the spinal cord: cellular mechanisms. Prog Neurobiol. 1998;54:349–365. - PubMed
-
- Beggs HE, Baragona SC, Hemperly JJ, Maness PF. NCAM140 interacts with the focal adhesion kinase p125(fak) and the SRC-related tyrosine kinase p59(fyn) J Biol Chem. 1997;272:8310–8319. - PubMed
-
- Bilderback TR, Gazula VR, Dobrowsky RT. Phosphoinositide 3-kinase regulates crosstalk between Trk A tyrosine kinase and p75(NTR)-dependent sphingolipid signaling pathways. J Neurochem. 2001;76:1540–1551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
