Sites and molecular mechanisms of modulation of Na(v)1.2 channels by Fyn tyrosine kinase
- PMID: 17959797
- PMCID: PMC6673227
- DOI: 10.1523/JNEUROSCI.1743-07.2007
Sites and molecular mechanisms of modulation of Na(v)1.2 channels by Fyn tyrosine kinase
Abstract
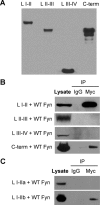
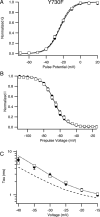
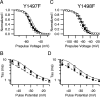
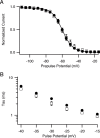
Voltage-gated sodium channels are important targets for modulation of electrical excitability by neurotransmitters and neurotrophins acting through protein phosphorylation. Fast inactivation of Na(V)1.2 channels is regulated via tyrosine phosphorylation by Fyn kinase and dephosphorylation by receptor phosphoprotein tyrosine phosphatase-beta, which are associated in a signaling complex. Here we have identified the amino acid residues on Na(V)1.2 channels that coordinate binding of Fyn kinase and mediate inhibition of sodium currents by enhancing fast inactivation. Fyn kinase binds to a Src homology 3 (SH3)-binding motif in the second half of the intracellular loop connecting domains I and II (L(I-II)) of Na(V)1.2, and mutation of that SH3-binding motif prevents Fyn binding and Fyn enhancement of fast inactivation of sodium currents. Analysis of tyrosine phosphorylation sites by mutagenesis and functional expression revealed a multisite regulatory mechanism. Y66 and Y1893, which are in consensus sequences appropriate for binding to the Fyn SH2 domain after phosphorylation, are both required for optimal binding and regulation by Fyn. Y730, which is located near the SH3-binding motif in L(I-II), and Y1497 and Y1498 in the inactivation gate in L(III-IV), are also required for optimal regulation. Phosphorylation of these sites likely promotes fast inactivation. Fast inactivation of the closely related Na(V)1.1 channels is not modulated by Fyn, and these channels do not contain an SH3-binding motif in L(I-II). Subtype-selective modulation by tyrosine phosphorylation/dephosphorylation provides a mechanism for differential regulation of sodium channels by neurotrophins and tyrosine phosphorylation in unmyelinated axons and dendrites, where Na(V)1.2 channels are expressed in brain neurons.
Figures




Similar articles
-
Regulation of Na(v)1.2 channels by brain-derived neurotrophic factor, TrkB, and associated Fyn kinase.J Neurosci. 2007 Oct 24;27(43):11533-42. doi: 10.1523/JNEUROSCI.5005-06.2007. J Neurosci. 2007. PMID: 17959796 Free PMC article.
-
Modulation of the cardiac sodium channel NaV1.5 by Fyn, a Src family tyrosine kinase.Circ Res. 2005 May 13;96(9):991-8. doi: 10.1161/01.RES.0000166324.00524.dd. Epub 2005 Apr 14. Circ Res. 2005. PMID: 15831816
-
Molecular mechanism of convergent regulation of brain Na(+) channels by protein kinase C and protein kinase A anchored to AKAP-15.Mol Cell Neurosci. 2002 Sep;21(1):63-80. doi: 10.1006/mcne.2002.1162. Mol Cell Neurosci. 2002. PMID: 12359152
-
Molecular properties of brain sodium channels: an important target for anticonvulsant drugs.Adv Neurol. 1999;79:441-56. Adv Neurol. 1999. PMID: 10514834 Review.
-
Advances in the expression and function of Fyn in different human tumors.Clin Transl Oncol. 2023 Oct;25(10):2852-2860. doi: 10.1007/s12094-023-03167-9. Epub 2023 Apr 24. Clin Transl Oncol. 2023. PMID: 37093456 Review.
Cited by
-
Localization of sodium channel subtypes in mouse ventricular myocytes using quantitative immunocytochemistry.J Mol Cell Cardiol. 2013 Nov;64:69-78. doi: 10.1016/j.yjmcc.2013.08.004. Epub 2013 Aug 24. J Mol Cell Cardiol. 2013. PMID: 23982034 Free PMC article.
-
Regulation of sodium channel activity by phosphorylation.Semin Cell Dev Biol. 2011 Apr;22(2):160-5. doi: 10.1016/j.semcdb.2010.10.002. Epub 2010 Oct 13. Semin Cell Dev Biol. 2011. PMID: 20950703 Free PMC article. Review.
-
SRC tyrosine kinases regulate neuronal differentiation of mouse embryonic stem cells via modulation of voltage-gated sodium channel activity.Neurochem Res. 2015 Apr;40(4):674-87. doi: 10.1007/s11064-015-1514-4. Epub 2015 Jan 11. Neurochem Res. 2015. PMID: 25577147
-
SRC-family tyrosine kinases in oogenesis, oocyte maturation and fertilization: an evolutionary perspective.Adv Exp Med Biol. 2014;759:33-56. doi: 10.1007/978-1-4939-0817-2_3. Adv Exp Med Biol. 2014. PMID: 25030759 Free PMC article. Review.
-
Regulation of voltage-gated sodium current by endogenous Src family kinases in cochlear spiral ganglion neurons in culture.Pflugers Arch. 2012 Apr;463(4):571-84. doi: 10.1007/s00424-012-1072-4. Pflugers Arch. 2012. PMID: 22297656
References
-
- Ahern CA, Zhang JF, Wookalis MJ, Horn R. Modulation of the cardiac sodium channel NaV1.5 by Fyn, a Src family tyrosine kinase. Circ Res. 2005;96:991–998. - PubMed
-
- Alland L, Peseckis SM, Atherton RE, Berthiaume L, Resh MD. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J Biol Chem. 1994;269:16701–16705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
