Role of extracellular sialic acid in regulation of neuronal and network excitability in the rat hippocampus
- PMID: 17959801
- PMCID: PMC6673228
- DOI: 10.1523/JNEUROSCI.2033-07.2007
Role of extracellular sialic acid in regulation of neuronal and network excitability in the rat hippocampus
Abstract
The extracellular membrane surface contains a substantial amount of negatively charged sialic acid residues. Some of the sialic acids are located close to the pore of voltage-gated channel, substantially influencing their gating properties. However, the role of sialylation of the extracellular membrane in modulation of neuronal and network activity remains primarily unknown. The level of sialylation is controlled by neuraminidase (NEU), the key enzyme that cleaves sialic acids. Here we show that NEU treatment causes a large depolarizing shift of voltage-gated sodium channel activation/inactivation and action potential (AP) threshold without any change in the resting membrane potential of hippocampal CA3 pyramidal neurons. Cleavage of sialic acids by NEU also reduced sensitivity of sodium channel gating and AP threshold to extracellular calcium. At the network level, exogenous NEU exerted powerful anticonvulsive action both in vitro and in acute and chronic in vivo models of epilepsy. In contrast, a NEU blocker (N-acetyl-2,3-dehydro-2-deoxyneuraminic acid) dramatically reduced seizure threshold and aggravated hippocampal seizures. Thus, sialylation appears to be a powerful mechanism to control neuronal and network excitability. We propose that decreasing the amount of extracellular sialic acid residues can be a useful approach to reduce neuronal excitability and serve as a novel therapeutic approach in the treatment of seizures.
Figures
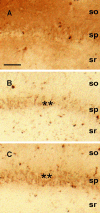

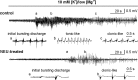
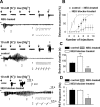
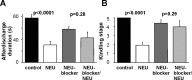
Comment in
-
In search of a new and improved target for antiepileptic drugs: sialic acid?Epilepsy Curr. 2008 May-Jun;8(3):79-80. doi: 10.1111/j.1535-7511.2008.00245.x. Epilepsy Curr. 2008. PMID: 18488062 Free PMC article. No abstract available.
Similar articles
-
The effect of neuraminidase blocker on gabazine-induced seizures in rat hippocampus.Fiziol Zh (1994). 2010;56(4):14-8. Fiziol Zh (1994). 2010. PMID: 20964139
-
Blockade of endogenous neuraminidase leads to an increase of neuronal excitability and activity-dependent synaptogenesis in the rat hippocampus.Eur J Neurosci. 2010 Dec;32(11):1889-96. doi: 10.1111/j.1460-9568.2010.07468.x. Epub 2010 Oct 29. Eur J Neurosci. 2010. PMID: 21044183
-
Differential sialylation modulates voltage-gated Na+ channel gating throughout the developing myocardium.J Gen Physiol. 2006 Mar;127(3):253-65. doi: 10.1085/jgp.200509423. Epub 2006 Feb 13. J Gen Physiol. 2006. PMID: 16476705 Free PMC article.
-
Modulation of voltage-gated ion channels by sialylation.Compr Physiol. 2012 Apr;2(2):1269-301. doi: 10.1002/cphy.c110044. Compr Physiol. 2012. PMID: 23798301 Review.
-
Epilepsy-associated alterations in hippocampal excitability.Rev Neurosci. 2017 Apr 1;28(3):307-334. doi: 10.1515/revneuro-2016-0059. Rev Neurosci. 2017. PMID: 28099137 Review.
Cited by
-
The role of Drosophila cytidine monophosphate-sialic acid synthetase in the nervous system.J Neurosci. 2013 Jul 24;33(30):12306-15. doi: 10.1523/JNEUROSCI.5220-12.2013. J Neurosci. 2013. PMID: 23884937 Free PMC article.
-
Sialic acids attached to N- and O-glycans within the Nav1.4 D1S5-S6 linker contribute to channel gating.Biochim Biophys Acta. 2015 Feb;1850(2):307-17. doi: 10.1016/j.bbagen.2014.10.027. Epub 2014 Oct 30. Biochim Biophys Acta. 2015. PMID: 25450184 Free PMC article.
-
Complete spatial characterisation of N-glycosylation upon striatal neuroinflammation in the rodent brain.J Neuroinflammation. 2021 May 16;18(1):116. doi: 10.1186/s12974-021-02163-6. J Neuroinflammation. 2021. PMID: 33993882 Free PMC article.
-
Surface expression and function of Cav3.2 T-type calcium channels are controlled by asparagine-linked glycosylation.Pflugers Arch. 2013 Aug;465(8):1159-70. doi: 10.1007/s00424-013-1259-3. Epub 2013 Mar 16. Pflugers Arch. 2013. PMID: 23503728
-
Regulated and aberrant glycosylation modulate cardiac electrical signaling.Proc Natl Acad Sci U S A. 2009 Sep 22;106(38):16517-22. doi: 10.1073/pnas.0905414106. Epub 2009 Aug 7. Proc Natl Acad Sci U S A. 2009. PMID: 19666501 Free PMC article.
References
-
- Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol. 2006;80:129–164. - PubMed
-
- Castillo C, Díaz ME, Balbi D, Thornhill WB, Recio-Pinto E. Changes in sodium channel function during postnatal brain development reflect increases in the level of channel sialylation. Dev Brain Res. 1997;104:119–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
