A role of the transient receptor potential domain of vanilloid receptor I in channel gating
- PMID: 17959807
- PMCID: PMC6673226
- DOI: 10.1523/JNEUROSCI.2457-07.2007
A role of the transient receptor potential domain of vanilloid receptor I in channel gating
Abstract
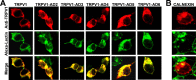
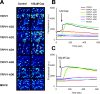
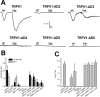
Transient receptor potential vanilloid receptor subtype 1 (TRPV1) is an ionotropic receptor activated by temperature and chemical stimuli. The C-terminal region that is adjacent to the channel gate, recognized as the TRP domain, is a molecular determinant of receptor assembly. However, the role of this intracellular domain in channel function remains elusive. Here, we show that replacement of the TRP domain of TRPV1 with the cognate region of TRPV channels (TRPV2-TRPV6) did not affect receptor assembly and trafficking to the cell surface, although those receptors containing the TRP domain of the distantly related TRPV5 and TRPV6 did not display ion channel activity. Notably, functional chimeras exhibited an impaired sensitivity to the activating stimuli, consistent with a significant contribution of this protein domain to channel function. At variance with TRPV1, voltage-dependent gating of chimeric channels could not be detected in the absence of capsaicin and/or heat. Biophysical analysis of functional chimeras revealed that the TRP domain appears to act as a molecular determinant of the activation energy of channel gating. Together, these findings uncover a role of the TRP domain in intersubunit interactions near the channel gate that contribute to the coupling of stimulus sensing to channel opening.
Figures


Similar articles
-
Mutation of I696 and W697 in the TRP box of vanilloid receptor subtype I modulates allosteric channel activation.J Gen Physiol. 2014 Mar;143(3):361-75. doi: 10.1085/jgp.201311070. J Gen Physiol. 2014. PMID: 24567510 Free PMC article.
-
The Integrity of the TRP Domain Is Pivotal for Correct TRPV1 Channel Gating.Biophys J. 2015 Aug 4;109(3):529-41. doi: 10.1016/j.bpj.2015.06.039. Biophys J. 2015. PMID: 26244735 Free PMC article.
-
The region adjacent to the C-end of the inner gate in transient receptor potential melastatin 8 (TRPM8) channels plays a central role in allosteric channel activation.J Biol Chem. 2014 Oct 10;289(41):28579-94. doi: 10.1074/jbc.M114.577478. Epub 2014 Aug 25. J Biol Chem. 2014. PMID: 25157108 Free PMC article.
-
ThermoTRP channels as modular proteins with allosteric gating.Cell Calcium. 2007 Oct-Nov;42(4-5):427-38. doi: 10.1016/j.ceca.2007.04.004. Epub 2007 May 17. Cell Calcium. 2007. PMID: 17499848 Review.
-
Beyond Hot and Spicy: TRPV Channels and their Pharmacological Modulation.Cell Physiol Biochem. 2021 May 28;55(S3):108-130. doi: 10.33594/000000358. Cell Physiol Biochem. 2021. PMID: 34043299 Review.
Cited by
-
Structure of the human lipid-gated cation channel TRPC3.Elife. 2018 May 4;7:e36852. doi: 10.7554/eLife.36852. Elife. 2018. PMID: 29726814 Free PMC article.
-
Decrypting the Heat Activation Mechanism of TRPV1 Channel by Molecular Dynamics Simulation.Biophys J. 2018 Jan 9;114(1):40-52. doi: 10.1016/j.bpj.2017.10.034. Biophys J. 2018. PMID: 29320695 Free PMC article.
-
A combined coarse-grained and all-atom simulation of TRPV1 channel gating and heat activation.J Gen Physiol. 2015 May;145(5):443-56. doi: 10.1085/jgp.201411335. J Gen Physiol. 2015. PMID: 25918362 Free PMC article.
-
TRPV1 in chronic pruritus and pain: Soft modulation as a therapeutic strategy.Front Mol Neurosci. 2022 Sep 2;15:930964. doi: 10.3389/fnmol.2022.930964. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36117910 Free PMC article. Review.
-
Mutation of I696 and W697 in the TRP box of vanilloid receptor subtype I modulates allosteric channel activation.J Gen Physiol. 2014 Mar;143(3):361-75. doi: 10.1085/jgp.201311070. J Gen Physiol. 2014. PMID: 24567510 Free PMC article.
References
-
- Arniges M, Fernandez-Fernandez JM, Albrecht N, Schaefer M, Valverde MA. Human TRPV4 channel splice variants revealed a key role of ankyrin domains in multimerization and trafficking. J Biol Chem. 2006;281:1580–1586. - PubMed
-
- Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, Patapoutian A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci. 2006;9:493–500. - PubMed
-
- Caprini M, Fava M, Valente P, Fernandez-Ballester G, Rapisarda C, Ferroni S, Ferrer-Montiel A. Molecular compatibility of the channel gate and the N terminus of S5 segment for voltage-gated channel activity. J Biol Chem. 2005;280:18253–18264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
