Mint3/X11gamma is an ADP-ribosylation factor-dependent adaptor that regulates the traffic of the Alzheimer's Precursor protein from the trans-Golgi network
- PMID: 17959829
- PMCID: PMC2174186
- DOI: 10.1091/mbc.e07-05-0465
Mint3/X11gamma is an ADP-ribosylation factor-dependent adaptor that regulates the traffic of the Alzheimer's Precursor protein from the trans-Golgi network
Abstract
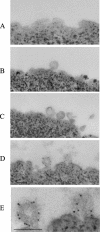
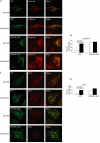
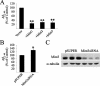
Beta-amyloid peptides (Abeta) are the major component of plaques in brains of Alzheimer's patients, and are they derived from the proteolytic processing of the beta-amyloid precursor protein (APP). The movement of APP between organelles is highly regulated, and it is tightly connected to its processing by secretases. We proposed previously that transport of APP within the cell is mediated in part through its sorting into Mint/X11-containing carriers. To test our hypothesis, we purified APP-containing vesicles from human neuroblastoma SH-SY5Y cells, and we showed that Mint2/3 are specifically enriched and that Mint3 and APP are present in the same vesicles. Increasing cellular APP levels increased the amounts of both APP and Mint3 in purified vesicles. Additional evidence supporting an obligate role for Mint3 in traffic of APP from the trans-Golgi network to the plasma membrane include the observations that depletion of Mint3 by small interference RNA (siRNA) or mutation of the Mint binding domain of APP changes the export route of APP from the basolateral to the endosomal/lysosomal sorting route. Finally, we show that increased expression of Mint3 decreased and siRNA-mediated knockdowns increased the secretion of the neurotoxic beta-amyloid peptide, Abeta(1-40). Together, our data implicate Mint3 activity as a critical determinant of post-Golgi APP traffic.
Figures





Similar articles
-
Recruitment of the Mint3 adaptor is necessary for export of the amyloid precursor protein (APP) from the Golgi complex.J Biol Chem. 2013 Oct 4;288(40):28567-80. doi: 10.1074/jbc.M113.481101. Epub 2013 Aug 21. J Biol Chem. 2013. PMID: 23965993 Free PMC article.
-
Amyloid precursor protein traffics from the Golgi directly to early endosomes in an Arl5b- and AP4-dependent pathway.Traffic. 2017 Mar;18(3):159-175. doi: 10.1111/tra.12465. Epub 2017 Jan 30. Traffic. 2017. PMID: 28000370
-
GGA1-mediated endocytic traffic of LR11/SorLA alters APP intracellular distribution and amyloid-β production.Mol Biol Cell. 2012 Jul;23(14):2645-57. doi: 10.1091/mbc.E12-01-0014. Epub 2012 May 23. Mol Biol Cell. 2012. PMID: 22621900 Free PMC article.
-
The GGA proteins: key players in protein sorting at the trans-Golgi network.Eur J Cell Biol. 2004 Jul;83(6):257-62. doi: 10.1078/0171-9335-00374. Eur J Cell Biol. 2004. PMID: 15511083 Review.
-
Cargo Sorting at the trans-Golgi Network for Shunting into Specific Transport Routes: Role of Arf Small G Proteins and Adaptor Complexes.Cells. 2019 Jun 3;8(6):531. doi: 10.3390/cells8060531. Cells. 2019. PMID: 31163688 Free PMC article. Review.
Cited by
-
Using Multi-Scale Genetic, Neuroimaging and Clinical Data for Predicting Alzheimer's Disease and Reconstruction of Relevant Biological Mechanisms.Sci Rep. 2018 Jul 24;8(1):11173. doi: 10.1038/s41598-018-29433-3. Sci Rep. 2018. PMID: 30042519 Free PMC article.
-
Trafficking in Alzheimer's Disease: Modulation of APP Transport and Processing by the Transmembrane Proteins LRP1, SorLA, SorCS1c, Sortilin, and Calsyntenin.Mol Neurobiol. 2018 Jul;55(7):5809-5829. doi: 10.1007/s12035-017-0806-x. Epub 2017 Oct 27. Mol Neurobiol. 2018. PMID: 29079999 Review.
-
Neurobeachin, a protein implicated in membrane protein traffic and autism, is required for the formation and functioning of central synapses.J Physiol. 2009 Nov 1;587(Pt 21):5095-106. doi: 10.1113/jphysiol.2009.178236. Epub 2009 Sep 1. J Physiol. 2009. PMID: 19723784 Free PMC article.
-
The trans-Golgi network is a major site for α-secretase processing of amyloid precursor protein in primary neurons.J Biol Chem. 2019 Feb 1;294(5):1618-1631. doi: 10.1074/jbc.RA118.005222. Epub 2018 Dec 13. J Biol Chem. 2019. PMID: 30545942 Free PMC article.
-
Cellular Trafficking of Amyloid Precursor Protein in Amyloidogenesis Physiological and Pathological Significance.Mol Neurobiol. 2019 Feb;56(2):812-830. doi: 10.1007/s12035-018-1106-9. Epub 2018 May 24. Mol Neurobiol. 2019. PMID: 29797184 Review.
References
-
- Andersen O. M., et al. Molecular dissection of the interaction between amyloid precursor protein and its neuronal trafficking receptor SorLA/LR11. Biochemistry. 2006;45:2618–2628. - PubMed
-
- Araki Y., Tomita S., Yamaguchi H., Miyagi N., Sumioka A., Kirino Y., Suzuki T. Novel cadherin-related membrane proteins, Alcadeins, enhance the X11-like protein-mediated stabilization of amyloid beta-protein precursor metabolism. J. Biol. Chem. 2003;278:49448–49458. - PubMed
-
- Bagshaw R. D., Pasternak S. H., Mahuran D. J., Callahan J. W. Nicastrin is a resident lysosomal membrane protein. Biochem. Biophys. Res. Commun. 2003;300:615–618. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

