Expansion of the nucleoplasmic reticulum requires the coordinated activity of lamins and CTP:phosphocholine cytidylyltransferase alpha
- PMID: 17959832
- PMCID: PMC2174170
- DOI: 10.1091/mbc.e07-02-0179
Expansion of the nucleoplasmic reticulum requires the coordinated activity of lamins and CTP:phosphocholine cytidylyltransferase alpha
Abstract
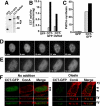
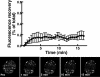
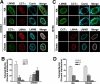
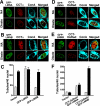
The nucleoplasmic reticulum (NR), a nuclear membrane network implicated in signaling and transport, is formed by the biosynthetic and membrane curvature-inducing properties of the rate-limiting enzyme in phosphatidylcholine synthesis, CTP:phosphocholine cytidylyltransferase (CCT) alpha. The NR is formed by invagination of the nuclear envelope and has an underlying lamina that may contribute to membrane tubule formation or stability. In this study we investigated the role of lamins A and B in NR formation in response to expression and activation of endogenous and fluorescent protein-tagged CCTalpha. Similarly to endogenous CCTalpha, CCT-green fluorescent protein (GFP) reversibly translocated to nuclear tubules projecting from the NE in response to oleate, a lipid promoter of CCT membrane binding. Coexpression and RNA interference experiments revealed that both CCTalpha and lamin A and B were necessary for NR proliferation. Expression of CCT-GFP mutants with compromised membrane-binding affinity produced fewer nuclear tubules, indicating that the membrane-binding function of CCTalpha promotes the expansion of the NR. Proliferation of atypical bundles of nuclear membrane tubules by a CCTalpha mutant that constitutively associated with membranes revealed that expansion of the double-bilayer NR requires the coordinated assembly of an underlying lamin scaffold and induction of membrane curvature by CCTalpha.
Figures


Similar articles
-
CTP:phosphocholine cytidylyltransferase α (CCTα) and lamins alter nuclear membrane structure without affecting phosphatidylcholine synthesis.Biochim Biophys Acta. 2011 Jun;1811(6):377-85. doi: 10.1016/j.bbalip.2011.04.001. Epub 2011 Apr 9. Biochim Biophys Acta. 2011. PMID: 21504799
-
Nuclear export of the rate-limiting enzyme in phosphatidylcholine synthesis is mediated by its membrane binding domain.J Lipid Res. 2009 May;50(5):966-76. doi: 10.1194/jlr.M800632-JLR200. Epub 2008 Dec 20. J Lipid Res. 2009. PMID: 19098306 Free PMC article.
-
The rate-limiting enzyme in phosphatidylcholine synthesis regulates proliferation of the nucleoplasmic reticulum.Mol Biol Cell. 2005 Mar;16(3):1120-30. doi: 10.1091/mbc.e04-10-0874. Epub 2005 Jan 5. Mol Biol Cell. 2005. PMID: 15635091 Free PMC article.
-
Regulation of CTP:phosphocholine cytidylyltransferase by amphitropism and relocalization.Trends Biochem Sci. 2000 Sep;25(9):441-7. doi: 10.1016/s0968-0004(00)01625-x. Trends Biochem Sci. 2000. PMID: 10973058 Review.
-
CTP:phosphocholine cytidylyltransferase: insights into regulatory mechanisms and novel functions.Biochem Biophys Res Commun. 1999 Apr 21;257(3):643-50. doi: 10.1006/bbrc.1999.0512. Biochem Biophys Res Commun. 1999. PMID: 10208837 Review.
Cited by
-
Pathological characteristics of axons and alterations of proteomic and lipidomic profiles in midbrain dopaminergic neurodegeneration induced by WDR45-deficiency.Mol Neurodegener. 2024 Aug 26;19(1):62. doi: 10.1186/s13024-024-00746-4. Mol Neurodegener. 2024. PMID: 39183331 Free PMC article.
-
14-3-3zeta escorts CCTalpha for calcium-activated nuclear import in lung epithelia.FASEB J. 2010 Apr;24(4):1271-83. doi: 10.1096/fj.09-136044. Epub 2009 Dec 9. FASEB J. 2010. PMID: 20007511 Free PMC article.
-
Differential contributions of phosphotransferases CEPT1 and CHPT1 to phosphatidylcholine homeostasis and lipid droplet biogenesis.J Biol Chem. 2023 Apr;299(4):104578. doi: 10.1016/j.jbc.2023.104578. Epub 2023 Mar 3. J Biol Chem. 2023. PMID: 36871755 Free PMC article.
-
Molecular mechanisms involved in farnesol-induced apoptosis.Cancer Lett. 2010 Jan 28;287(2):123-35. doi: 10.1016/j.canlet.2009.05.015. Epub 2009 Jun 10. Cancer Lett. 2010. PMID: 19520495 Free PMC article. Review.
-
Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling.Cell. 2012 May 11;149(4):832-46. doi: 10.1016/j.cell.2012.03.032. Cell. 2012. PMID: 22579286 Free PMC article.
References
-
- Bonne G., et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet. 1999;21:285–288. - PubMed
-
- Brandt A., et al. Developmental control of nuclear size and shape by Kugelkern and Kurzkern. Curr. Biol. 2006;16:543–552. - PubMed
-
- Broers J. L., Machiels B. M., van Eys G. J., Kuijpers H. J., Manders E. M., van Driel R., Ramaekers F. C. Dynamics of the nuclear lamina as monitored by GFP-tagged A-type lamins. J. Cell Sci. 1999;112(Pt 20):3463–3475. - PubMed
-
- Cornell R. Chemical cross-linking reveals a dimeric structure for CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 1989;264:9077–9082. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

