An Hfq-like protein in archaea: crystal structure and functional characterization of the Sm protein from Methanococcus jannaschii
- PMID: 17959927
- PMCID: PMC2080587
- DOI: 10.1261/rna.689007
An Hfq-like protein in archaea: crystal structure and functional characterization of the Sm protein from Methanococcus jannaschii
Abstract
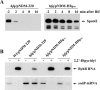
The Sm and Sm-like proteins are conserved in all three domains of life and have emerged as important players in many different RNA-processing reactions. Their proposed role is to mediate RNA-RNA and/or RNA-protein interactions. In marked contrast to eukaryotes, bacteria appear to contain only one distinct Sm-like protein belonging to the Hfq family of proteins. Similarly, there are generally only one or two subtypes of Sm-related proteins in archaea, but at least one archaeon, Methanococcus jannaschii, encodes a protein that is related to Hfq. This archaeon does not contain any gene encoding a conventional archaeal Sm-type protein, suggesting that Hfq proteins and archaeal Sm-homologs can complement each other functionally. Here, we report the functional characterization of M. jannaschii Hfq and its crystal structure at 2.5 A resolution. The protein forms a hexameric ring. The monomer fold, as well as the overall structure of the complex is similar to that found for the bacterial Hfq proteins. However, clear differences are seen in the charge distribution on the distal face of the ring, which is unusually negative in M. jannaschii Hfq. Moreover, owing to a very short N-terminal alpha-helix, the overall diameter of the archaeal Hfq hexamer is significantly smaller than its bacterial counterparts. Functional analysis reveals that Escherichia coli and M. jannaschii Hfqs display very similar biochemical and biological properties. It thus appears that the archaeal and bacterial Hfq proteins are largely functionally interchangeable.
Figures

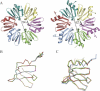




References
-
- Adams, P.D., Grosse-Kunstleve, R.W., Hung, L.-W., Ioerger, T.R., McCoy, A.J., Moriarty, N.W., Read, R.J., Sacchettini, J.C., Sauter, N.K., Terwilliger, T.C. PHENIX: Building new software for automated crystallographic structure determination. Acta Crystallogr. 2002;D58:1948–1954. - PubMed
-
- Aiba, H. Mechanism of RNA silencing by Hfq-binding small RNAs. Curr. Opin. Microbiol. 2007;10:134–139. - PubMed
-
- Beggs, J. Lsm proteins and RNA processing. Biochem. Soc. Trans. 2005;33:433–438. - PubMed
-
- Brennan, R.G., Link, T.M. Hfq structure, function and ligand binding. Curr. Opin. Microbiol. 2007;10:125–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
