Functional analysis of polyphenol oxidases by antisense/sense technology
- PMID: 17960074
- PMCID: PMC6149088
- DOI: 10.3390/12081569
Functional analysis of polyphenol oxidases by antisense/sense technology
Abstract
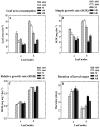
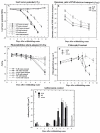
Polyphenol oxidases (PPOs) catalyze the oxidation of phenolics to quinones, the secondary reactions of which lead to oxidative browning and postharvest losses of many fruits and vegetables. PPOs are ubiquitous in angiosperms, are inducible by both biotic and abiotic stresses, and have been implicated in several physiological processes including plant defense against pathogens and insects, the Mehler reaction, photoreduction of molecular oxygen by PSI, regulation of plastidic oxygen levels, aurone biosynthesis and the phenylpropanoid pathway. Here we review experiments in which the roles of PPO in disease and insect resistance as well as in the Mehler reaction were investigated using transgenic tomato (Lycopersicon esculentum) plants with modified PPO expression levels (suppressed PPO and overexpressing PPO). These transgenic plants showed normal growth, development and reproduction under laboratory, growth chamber and greenhouse conditions. Antisense PPO expression dramatically increased susceptibility while PPO overexpression increased resistance of tomato plants to Pseudomonas syringae. Similarly, PPO-overexpressing transgenic plants showed an increase in resistance to various insects, including common cutworm (Spodoptera litura (F.)), cotton bollworm (Helicoverpa armigera (Hübner)) and beet army worm (Spodoptera exigua (Hübner)), whereas larvae feeding on plants with suppressed PPO activity had higher larval growth rates and consumed more foliage. Similar increases in weight gain, foliage consumption, and survival were also observed with Colorado potato beetles (Leptinotarsa decemlineata (Say)) feeding on antisense PPO transgenic tomatoes. The putative defensive mechanisms conferred by PPO and its interaction with other defense proteins are discussed. In addition, transgenic plants with suppressed PPO exhibited more favorable water relations and decreased photoinhibition compared to nontransformed controls and transgenic plants overexpressing PPO, suggesting that PPO may have a role in the development of plant water stress and potential for photoinhibition and photooxidative damage that may be unrelated to any effects on the Mehler reaction. These results substantiate the defensive role of PPO and suggest that manipulation of PPO activity in specific tissues has the potential to provide broad-spectrum resistance simultaneously to both disease and insect pests, however, effects of PPO on postharvest quality as well as water stress physiology should also be considered. In addition to the functional analysis of tomato PPO, the application of antisense/sense technology to decipher the functions of PPO in other plant species as well as for commercial uses are discussed.
Figures



References
-
- Mayer A. M., Harel E. Phenoloxidases and Their Significance in Fruit and Vegetables. In: Fox P. F., editor. Food Enzymology. Elsevier; New York: 1991. pp. 373–398.
- Friedman M. Chemistry, Biochemistry, and Dietary Role of Potato Polyphenols. J. Agric. Food Chem. 1997;45:1523–1540.
-
- Kojima M., Takeuchi W. Detection and Characterization of p-Coumaric Acid Hydroxylase in Mungbean, Vigna mungo, Seedlings. J. Biochem. 1989;105:265–270. - PubMed
-
- Vaughn K. C., Lax A. R., Duke S. O. Polyphenol Oxidase: The Chloroplast Oxidase With No Established Function. Physiol. Plant. 1988;72:659–665.
- Trebst A., Depka B. Polyphenol Oxidase and Photosynthesis Research. Photosynth. Res. 1995;46:41–44. - PubMed
-
- Nakayama T., Yonekura-Sakakibara K., Sato T., Kikuchi S., Fukui Y., Fukuchi-Mizutani M., Ueda T., Nakao M., Tanaka Y., Kusumi T., Nishino T. Aureusidin Synthase: A Polyphenol Oxidase Homolog Responsible for Flower Coloration. Science. 2000;290:1163–1166. doi: 10.1126/science.290.5494.1163. - DOI - PubMed
-
- Felton G. W., Donato K. K., Del Vecchio R. J., Duffey S. S. Activation of Plant Foliar Oxidases by Insect Feeding Reduces Nutritive Quality of Foliage for Noctuid Herbivores. J. Chem. Ecol. 1989;15:2667–2694. - PubMed
- Stout M. J., Workman K. V., Bostock R. M., Duffey S. S. Stimulation and Attenuation of Induced Resistance by Elicitors and Inhibitors of Chemical Induction in Tomato (Lycopersicon esculentum) Foliage. Entomol. Exper. Appli. 1998;86:267–279.
- Li L., Steffens J. C. Overexpression of Polyphenol Oxidase in Transgenic Tomato Plants Results in Enhanced Bacterial Disease Resistance. Planta. 2002;215:239–247. - PubMed
- Thipyapong P., Hunt M. D., Steffens J. C. Antisense Downregulation of Polyphenol Oxidase Results in Enhanced Disease Susceptibility. Planta. 2004;220:105–117. - PubMed
- Wang J., Constabel C. P. Polyphenol Oxidase Overexpression in Transgenic Populus Enhances Resistance to Herbivory by Forest Tent Caterpillar (Malacosoma disstria) Planta. 2004;220:87–96. - PubMed
- Barbehenn R. V., Jones C. P., Yip L., Tran L., Constabel C. P. Does the Induction of Polyphenol Oxidase Defend Trees against Caterpillars? Assessing Plant Defenses One at a Time with Transgenic Poplar. Oecologia. 2007;220 in press. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

