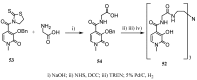
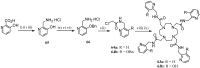
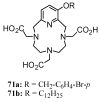
Synthetic approaches to heterocyclic ligands for Gd-based MRI contrast agents
- PMID: 17960087
- PMCID: PMC6149094
- DOI: 10.3390/12081771
Synthetic approaches to heterocyclic ligands for Gd-based MRI contrast agents
Abstract
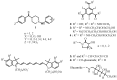
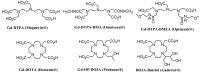
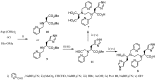
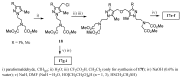
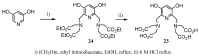
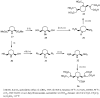
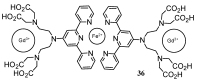
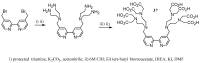
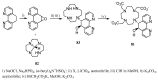
Magnetic Resonance Imaging (MRI) methods are currently used in the clinic for the non invasive detection and characterization of a wide variety of pathologies. Increases in the diagnostic efficiency of MRI have been helped by both the design of dedicated MR sequences revealing specific aspects of the pathology and by the development of more sensitive and selective Contrast Agents (CAs), capable of more precisely delineating the borderline regions. In the present review we focus on the synthetic strategies used to obtain MRI CAs containing heterocyclic rings.
Figures

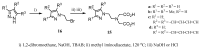
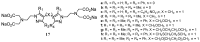

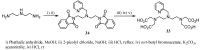
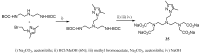

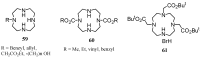




Similar articles
-
Gd-TEMDO: Design, Synthesis, and MRI Application.Chemistry. 2016 May 23;22(22):7352-6. doi: 10.1002/chem.201600720. Epub 2016 Apr 8. Chemistry. 2016. PMID: 26991633 Free PMC article.
-
Synthesis and relaxivity studies of a tetranuclear gadolinium(III) complex of DO3A as a contrast-enhancing agent for MRI.Inorg Chem. 2005 Dec 12;44(25):9434-43. doi: 10.1021/ic050743r. Inorg Chem. 2005. PMID: 16323930
-
Cyclen-based Gd3+ complexes as MRI contrast agents: Relaxivity enhancement and ligand design.Bioorg Med Chem. 2016 Nov 15;24(22):5663-5684. doi: 10.1016/j.bmc.2016.09.069. Epub 2016 Oct 1. Bioorg Med Chem. 2016. PMID: 27729196 Review.
-
[Gd-AAZTA]-: a new structural entry for an improved generation of MRI contrast agents.Inorg Chem. 2004 Nov 29;43(24):7588-90. doi: 10.1021/ic0489692. Inorg Chem. 2004. PMID: 15554621
-
Physicochemical properties of gadoteridol and other magnetic resonance contrast agents.Invest Radiol. 1992 Aug;27 Suppl 1:S2-6. Invest Radiol. 1992. PMID: 1506149 Review. No abstract available.

