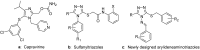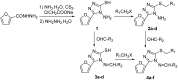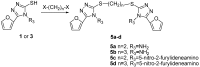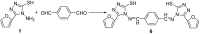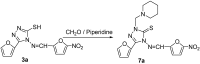Synthesis of novel derivatives of 4-amino-3-(2-furyl)-5-mercapto-1,2,4-triazole as potential HIV-1 NNRTIs
- PMID: 17960101
- PMCID: PMC6149092
- DOI: 10.3390/12082003
Synthesis of novel derivatives of 4-amino-3-(2-furyl)-5-mercapto-1,2,4-triazole as potential HIV-1 NNRTIs
Abstract
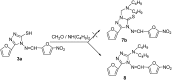
A series of 5-alkylthio (2a-d), 4-arylideneamino (3a-d) and 4-arylideneamino-5-alkylthio derivatives (4a-f) of 4-amino-3-(2-furyl)-5-mercapto-1,2,4-triazole (1) were synthesized by alkylation of the parent compound with alkyl halides and condensation with aldehydes, respectively. Sulfanyl dimers 5a-d and 4-iminomethyl dimer 6 were correspondingly prepared by reaction with alkane dibromides and 1,4-diformylbenzene. Mannich base 7 was also synthesized by aminomethylation of the 3-sulfanyltriazole 1 at the N1 position. The newly designed and synthesized substituted s-triazole derivatives were assayed for anti-HIV-1 activity by examination of their inhibition of HIV-1-induced cytopathogenicity in MT-4 cells and by determination of their inhibitory effect on HIV-1 reverse transcriptase. Compound 4e was found to be the most active inhibitor against HIV-1 replication in cell culture (EC50 = 12 microM) and against HIV-1 reverse transcriptase (IC50 = 43.5 microM), which provided a good lead for further optimization.
Figures
Similar articles
-
Triazole derivatives as non-nucleoside inhibitors of HIV-1 reverse transcriptase--structure-activity relationships and crystallographic analysis.Bioorg Med Chem Lett. 2008 Feb 1;18(3):1131-4. doi: 10.1016/j.bmcl.2007.11.127. Epub 2007 Dec 5. Bioorg Med Chem Lett. 2008. PMID: 18083512
-
Design, synthesis, and anti-HIV-1 activity of 1-aromatic methyl-substituted 3-(3,5-dimethylbenzyl)uracil and N-3,5-dimethylbenzyl-substituted urea derivatives.Antivir Chem Chemother. 2015 Feb;24(1):3-18. doi: 10.1177/2040206614566584. Antivir Chem Chemother. 2015. PMID: 26149262 Free PMC article.
-
Arylazolylthioacetanilide. Part 8: Design, synthesis and biological evaluation of novel 2-(2-(2,4-dichlorophenyl)-2H-1,2,4-triazol-3-ylthio)-N-arylacetamides as potent HIV-1 inhibitors.Eur J Med Chem. 2011 Oct;46(10):5039-45. doi: 10.1016/j.ejmech.2011.08.011. Epub 2011 Aug 12. Eur J Med Chem. 2011. PMID: 21872971
-
Design, synthesis and biological evaluation of novel acetamide-substituted doravirine and its prodrugs as potent HIV-1 NNRTIs.Bioorg Med Chem. 2019 Feb 1;27(3):447-456. doi: 10.1016/j.bmc.2018.12.039. Epub 2018 Dec 30. Bioorg Med Chem. 2019. PMID: 30606670 Review.
-
Sulfanyltriazole/tetrazoles: a promising class of HIV-1 NNRTIs.Mini Rev Med Chem. 2009 Jul;9(8):1014-23. doi: 10.2174/138955709788681618. Mini Rev Med Chem. 2009. PMID: 19601897 Review.
Cited by
-
Detection of a target protein (GroEl2) in Mycobacterium tuberculosis using a derivative of 1,2,4-triazolethiols.Mol Divers. 2022 Oct;26(5):2535-2548. doi: 10.1007/s11030-021-10351-y. Epub 2021 Nov 25. Mol Divers. 2022. PMID: 34822095
-
Two decades of the synthesis of mono- and bis-aminomercapto[1,2,4]triazoles.RSC Adv. 2020 Jul 1;10(42):24994-25012. doi: 10.1039/d0ra04208k. eCollection 2020 Jun 29. RSC Adv. 2020. PMID: 35517465 Free PMC article. Review.
-
Synthesis and anti-human immunodeficiency virus type 1 activity of (E)-N-phenylstyryl-N-alkylacetamide derivatives.Molecules. 2009 Aug 26;14(9):3176-86. doi: 10.3390/molecules14093176. Molecules. 2009. PMID: 19783916 Free PMC article.
References
-
- Wainberg M. A., Sawyer J. P., Montaner J. S., Murphy R. L., Kuritzkes D. R., Raffi F. Challenges for the clinical development of new nucleoside reverse transcriptase inhibitors for HIV infection. Antivir. Ther. 2005;10:13–28. - PubMed
-
- Di Santo R., Costi R., Artico M., Miele G., Lavecchia A., Novellino E., Bergamini A., Cancio R., Maga G. Arylthiopyrrole (AThP) derivatives as non-nucleoside HIV-1 reverse transcriptase inhibitors: synthesis, structure-activity relationships, and docking studies (part 1) Chem. Med. Chem. 2006;1:1367–1378. doi: 10.1002/cmdc.200600119. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical