Homeodomain-interacting protein kinase-2 (HIPK2) phosphorylates HMGA1a at Ser-35, Thr-52, and Thr-77 and modulates its DNA binding affinity
- PMID: 17960875
- PMCID: PMC2547408
- DOI: 10.1021/pr700571d
Homeodomain-interacting protein kinase-2 (HIPK2) phosphorylates HMGA1a at Ser-35, Thr-52, and Thr-77 and modulates its DNA binding affinity
Abstract
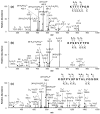
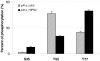
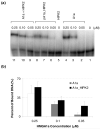
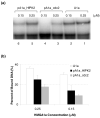
The chromosomal high-mobility group A (HMGA) proteins, composed of HMGA1a, HMGA1b and HMGA2, play important roles in the regulation of numerous processes in eukaryotic cells, such as transcriptional regulation, DNA repair, RNA processing, and chromatin remodeling. The biological activities of HMGA1 proteins are highly regulated by their post-translational modifications (PTMs), including acetylation, methylation, and phosphorylation. Recently, it was found that the homeodomain-interacting protein kinase-2 (HIPK2), a newly identified serine/threonine kinase, co-immunoprecipitated with, and phosphorylated, HMGA1 proteins. However, the sites and the biological significance of the phosphorylation have not been elucidated. Here, we found that HIPK2 phosphorylates HMGA1a at Ser-35, Thr-52, and Thr-77, and HMGA1b at Thr-41 and Thr-66. In addition, we demonstrated that cdc2, which is known to phosphorylate HMGA1 proteins, could induce the phosphorylation of HMGA1 proteins at the same Ser/Thr sites. The two kinases, however, exhibited different site preferences for the phosphorylation: The preference for HIPK2 phosphorylation followed the order of Thr-77 > Thr-52 > Ser-35, whereas the order for cdc2 phosphorylation was Thr-52 > Thr-77 > Ser-35. Moreover, we found that the HIPK2-phosphorylated HMGA1a reduced the binding affinity of HMGA1a to human germ line promoter, and the drop in binding affinity induced by HIPK2 phosphorylation was lower than that introduced by cdc2 phosphorylation, which is consistent with the notion that the second AT-hook in HMGA1a is more important for DNA binding than the third AT-hook.
Figures
Similar articles
-
A mass spectrometric study on the in vitro methylation of HMGA1a and HMGA1b proteins by PRMTs: methylation specificity, the effect of binding to AT-rich duplex DNA, and the effect of C-terminal phosphorylation.Biochemistry. 2007 Jul 3;46(26):7896-906. doi: 10.1021/bi6024897. Epub 2007 Jun 6. Biochemistry. 2007. PMID: 17550233
-
Dynamic and differential in vivo modifications of the isoform HMGA1a and HMGA1b chromatin proteins.J Biol Chem. 2005 Mar 11;280(10):8961-73. doi: 10.1074/jbc.M407348200. Epub 2004 Dec 11. J Biol Chem. 2005. PMID: 15591590
-
Tandem mass spectrometry for the examination of the posttranslational modifications of high-mobility group A1 proteins: symmetric and asymmetric dimethylation of Arg25 in HMGA1a protein.Biochemistry. 2005 Apr 26;44(16):6293-301. doi: 10.1021/bi0475525. Biochemistry. 2005. PMID: 15835918
-
HMGA1-pseudogenes and cancer.Oncotarget. 2016 May 10;7(19):28724-35. doi: 10.18632/oncotarget.7427. Oncotarget. 2016. PMID: 26895108 Free PMC article. Review.
-
Posttranslational modifications regulate HIPK2, a driver of proliferative diseases.J Mol Med (Berl). 2013 Sep;91(9):1051-8. doi: 10.1007/s00109-013-1042-0. Epub 2013 Apr 25. J Mol Med (Berl). 2013. PMID: 23616089 Review.
Cited by
-
HMGA proteins as modulators of chromatin structure during transcriptional activation.Front Cell Dev Biol. 2014 Mar 6;2:5. doi: 10.3389/fcell.2014.00005. eCollection 2014. Front Cell Dev Biol. 2014. PMID: 25364713 Free PMC article. Review.
-
The dynamics of HMG protein-chromatin interactions in living cells.Biochem Cell Biol. 2009 Feb;87(1):127-37. doi: 10.1139/O08-110. Biochem Cell Biol. 2009. PMID: 19234529 Free PMC article. Review.
-
An RNA interference screen identifies druggable regulators of MeCP2 stability.Sci Transl Med. 2017 Aug 23;9(404):eaaf7588. doi: 10.1126/scitranslmed.aaf7588. Sci Transl Med. 2017. PMID: 28835516 Free PMC article.
-
Phosphoproteomics profiling reveals a kinase network conferring acute myeloid leukaemia intrinsic chemoresistance and indicates HMGA1 phosphorylation as a potential influencer.Clin Transl Med. 2022 Mar;12(3):e749. doi: 10.1002/ctm2.749. Clin Transl Med. 2022. PMID: 35297189 Free PMC article. No abstract available.
-
The High Mobility Group A1 (HMGA1) Transcriptome in Cancer and Development.Curr Mol Med. 2016;16(4):353-93. doi: 10.2174/1566524016666160316152147. Curr Mol Med. 2016. PMID: 26980699 Free PMC article. Review.
References
-
- Bustin M, Reeves R. High-mobility-group chromosomal proteins: architectural components that facilitate chromatin function. Prog. Nucleic Acid Res. Mol. Biol. 1996;54:35–100. - PubMed
-
- Sgarra R, Rustighi A, Tessari MA, Di Bernardo J, Altamura S, Fusco A, Manfioletti G, Giancotti V. Nuclear phosphoproteins HMGA and their relationship with chromatin structure and cancer. FEBS Lett. 2004;574:1–8. - PubMed
-
- Reeves R. Molecular biology of HMGA proteins: hubs of nuclear function. Gene. 2001;277:63–81. - PubMed
-
- Fedele M, Bandiera A, Chiappetta G, Battista S, Viglietto G, Manfioletti G, Casamassimi A, Santoro M, Giancotti V, Fusco A. Human colorectal carcinomas express high levels of high mobility group HMGI(Y) proteins. Cancer Res. 1996;56:1896–1901. - PubMed
-
- Bandiera A, Bonifacio D, Manfioletti G, Mantovani F, Rustighi A, Zanconati F, Fusco A, Di Bonito L, Giancotti V. Expression of HMGI(Y) proteins in squamous intraepithelial and invasive lesions of the uterine cervix. Cancer Res. 1998;58:426–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

