The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis
- PMID: 17961066
- PMCID: PMC2737712
- DOI: 10.1089/ars.2007.1897
The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis
Abstract
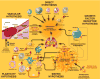
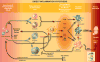
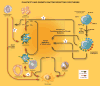
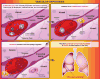
The role of inflammation in idiopathic pulmonary fibrosis (IPF) is controversial. If inflammation were critical to the disease process, lung pathology would demonstrate an influx of inflammatory cells, and that the disease would respond to immunosuppression. Neither is true. The classic pathology does not display substantial inflammation, and no modulation of the immune system is effective as treatment. Recent data suggest that the pathophysiology of the disease is more a product of fibroblast dysfunction than of dysregulated inflammation. The role of inflammation in disease pathogenesis comes from pathology from atypical patients, biologic samples procured during exacerbations of the disease, and careful examination of biologic specimens from patients with stable disease. We suggest that inflammation is indeed a critical factor in IPF and propose five potential nontraditional mechanisms for the role of inflammation in the pathogenesis of IPF: the direct inflammatory hypothesis, the matrix hypothesis, the growth factor-receptor hypothesis, the plasticity hypothesis, and the vascular hypothesis. To address these, we review the literature exploring the differences in pathology, prognosis, and clinical course, as well as the role of cytokines, growth factors, and other mediators of inflammation, and last, the role of matrix and vascular supply in patients with IPF.
Figures

References
-
- American Thoracic Society Committee of the Scientific Assembly on Environmental and Occupational Health. Adverse effects of crystalline silica exposure. Am J Respir Crit Care Med. 1997;155:761–768. - PubMed
-
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment: international consensus statement: American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. - PubMed
-
- American Thoracic Society/European Respiratory Society. International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias: this joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS Board of Directors, June 2001 and by The ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

