Extracellular superoxide dismutase protects against matrix degradation of heparan sulfate in the lung
- PMID: 17961072
- PMCID: PMC2289772
- DOI: 10.1089/ars.2007.1906
Extracellular superoxide dismutase protects against matrix degradation of heparan sulfate in the lung
Abstract
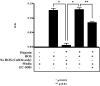
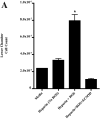
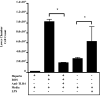
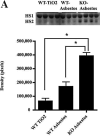
Asbestosis is a form of interstitial lung disease caused by the inhalation of asbestos fibers, leading to inflammation and pulmonary fibrosis. Inflammation and oxidant/antioxidant imbalances are known to contribute to the disease pathogenesis. Extracellular superoxide dismutase (EC-SOD) is an antioxidant enzyme that has been shown to protect the lung from oxidant-mediated damage, inflammation, and interstitial fibrosis. Extracellular matrix (ECM) components, such as collagen and glycosaminoglycans, are known to be sensitive to oxidative fragmentation. Heparan sulfate, a glycosaminoglycan, is highly abundant in the ECM and tightly binds EC-SOD. We investigated the protective role of EC-SOD by evaluating the interaction of EC-SOD with heparan sulfate in the presence of reactive oxygen species (ROS). We found that ROS-induced heparin and heparan sulfate fragments induced neutrophil chemotaxis across a modified Boyden chamber, which was inhibited by the presence of EC-SOD by scavenging oxygen radicals. Chemotaxis in response to oxidatively fragmented heparin was mediated by Toll-like receptor-4. In vivo, bronchoalveolar lavage fluid from EC-SOD knockout mice at 1, 14, and 28 days after asbestos exposure showed increased heparan sulfate shedding from the lung parenchyma. We demonstrate that one mechanism through which EC-SOD inhibits lung inflammation and fibrosis in asbestosis is by protecting heparin/heparan sulfate from oxidative fragmentation.
Figures




Similar articles
-
Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan.J Biol Chem. 2008 Mar 7;283(10):6058-66. doi: 10.1074/jbc.M709273200. Epub 2007 Dec 28. J Biol Chem. 2008. PMID: 18165226 Free PMC article.
-
Redistribution of pulmonary EC-SOD after exposure to asbestos.J Appl Physiol (1985). 2004 Nov;97(5):2006-13. doi: 10.1152/japplphysiol.00480.2004. Epub 2004 Aug 6. J Appl Physiol (1985). 2004. PMID: 15298984
-
Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis.J Biol Chem. 2009 Feb 6;284(6):3537-45. doi: 10.1074/jbc.M807001200. Epub 2008 Dec 9. J Biol Chem. 2009. PMID: 19073610 Free PMC article.
-
Extracellular superoxide dismutase in pulmonary fibrosis.Antioxid Redox Signal. 2008 Feb;10(2):343-54. doi: 10.1089/ars.2007.1908. Antioxid Redox Signal. 2008. PMID: 17999630 Free PMC article. Review.
-
Heparin-derived heparan sulfate mimics to modulate heparan sulfate-protein interaction in inflammation and cancer.Matrix Biol. 2010 Jul;29(6):442-52. doi: 10.1016/j.matbio.2010.04.003. Epub 2010 Apr 21. Matrix Biol. 2010. PMID: 20416374 Free PMC article. Review.
Cited by
-
The dynamic uptake and release of SOD3 from intracellular stores in macrophages modulates the inflammatory response.Redox Biol. 2019 Sep;26:101268. doi: 10.1016/j.redox.2019.101268. Epub 2019 Jul 2. Redox Biol. 2019. PMID: 31326693 Free PMC article.
-
Extracellular superoxide dismutase polymorphism in mice: Allele-specific effects on phenotype.Free Radic Biol Med. 2010 Feb 15;48(4):590-6. doi: 10.1016/j.freeradbiomed.2009.12.003. Epub 2009 Dec 11. Free Radic Biol Med. 2010. PMID: 20005946 Free PMC article.
-
Overexpression of Extracellular Superoxide Dismutase 3 Inhibits Cancer Cell Growth and Migration in Colorectal Cancer.Turk J Gastroenterol. 2024 Jun;35(6):465-474. doi: 10.5152/tjg.2024.23232. Turk J Gastroenterol. 2024. PMID: 39128081 Free PMC article.
-
Genetic and epigenetic inactivation of extracellular superoxide dismutase promotes an invasive phenotype in human lung cancer by disrupting ECM homeostasis.Mol Cancer Res. 2012 Jan;10(1):40-51. doi: 10.1158/1541-7786.MCR-11-0501. Epub 2011 Nov 7. Mol Cancer Res. 2012. PMID: 22064654 Free PMC article.
-
Manganese and extracellular superoxide dismutase polymorphisms and risk for asbestosis.J Biomed Biotechnol. 2009;2009:493083. doi: 10.1155/2009/493083. Epub 2009 Jul 14. J Biomed Biotechnol. 2009. PMID: 19636420 Free PMC article.
References
-
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. - PubMed
-
- Bowler RP, Crapo JD. Oxidative stress in airways: Is there a role for extracellular superoxide dismutase? Am J Respir Crit Care Med. 2002;166:S38–S43. - PubMed
-
- Fattman CL, Chu CT, Kulich SM, Enghild JJ, Oury TD. Altered expression of extracellular superoxide dismutase in mouse lung after bleomycin treatment. Free Radic Biol Med. 2001;31:1198–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

