Population pharmacokinetics of gemcitabine and its metabolite in patients with cancer: effect of oxaliplatin and infusion rate
- PMID: 17961191
- PMCID: PMC2291243
- DOI: 10.1111/j.1365-2125.2007.03040.x
Population pharmacokinetics of gemcitabine and its metabolite in patients with cancer: effect of oxaliplatin and infusion rate
Abstract
What is already known about this subject. Gemcitabine is an anticancer drug which is metabolized to a number of metabolites, administered using different dosing regimens and increasingly used in combination with oxaliplatin. the impact of dosing strategies and combination therapy on the pharmacokinetics of gemcitabine and its main metabolite is not clearly understood. what this study adds. this study has characterized the pharmacokinetics of gemcitabine and its main metabolite in people with cancer, including the variability between patients and on different occasions. gemcitabine metabolite (but not gemcitabine) pharmacokinetics were significantly affected by co-administration with oxaliplatin and were dependent on the order of administration. the clinical implications of this observation remain to be established.
Aims: To characterize the population pharmacokinetics of gemcitabine and its metabolite (dfdu) in patients with cancer and identify factors that are influential in gemcitabine dose regimen design.
Methods: Gemcitabine and dfdu plasma concentration-time and clinical data from 94 patients with cancer and nonlinear mixed effect modelling were used to characterize gemcitabine and metabolite pharmacokinetic variability and identify influential covariates.
Results: Gemcitabine and dFdU pharmacokinetics were described by a two-compartment model with first-order elimination. The population mean (and between-subject variability, CV%) for clearance and volume of distribution of the central compartment (V(C)) for gemcitabine were 2.7 l min(-1) (31%) and 15 l (39%), respectively, and 0.04 l min(-1) (35%) and 46 l (15%), respectively, for dFdU. Oxaliplatin co-administration significantly decreased dFdU V(C) by 35% when gemcitabine was administered first and by 46% when oxaliplatin was administered first compared with patients who received gemcitabine alone.
Conclusions: Co-administration of gemcitabine with oxaliplatin significantly affected the pharmacokinetics of dFdU. The clinical significance of this observation in the context of gemcitabine safety and efficacy is worthy of further investigation.
Figures


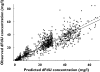

 ), 50th percentile (
), 50th percentile ( ), 95th percentile (
), 95th percentile ( ), Observed (○)
), Observed (○)
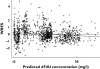
 ), 50th percentile (
), 50th percentile ( ), 95th percentile (
), 95th percentile ( ), Observed (○)
), Observed (○)Similar articles
-
Population pharmacokinetics of gemcitabine and its metabolite in Japanese cancer patients: impact of genetic polymorphisms.Clin Pharmacokinet. 2010 Aug;49(8):549-58. doi: 10.2165/11532970-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20608756
-
[Population pharmacokinetics of gemcitabine applied to personalize the dosage used in cancer patients].Farm Hosp. 2012 Jul-Aug;36(4):194-206. doi: 10.1016/j.farma.2011.04.002. Epub 2011 Nov 10. Farm Hosp. 2012. PMID: 22078546 Spanish.
-
SLC28A3 genotype and gemcitabine rate of infusion affect dFdCTP metabolite disposition in patients with solid tumours.Br J Cancer. 2014 Jan 21;110(2):304-12. doi: 10.1038/bjc.2013.738. Epub 2013 Dec 3. Br J Cancer. 2014. PMID: 24300978 Free PMC article.
-
Is age just a number? A population pharmacokinetic study of gemcitabine.Cancer Chemother Pharmacol. 2022 May;89(5):697-705. doi: 10.1007/s00280-022-04431-5. Epub 2022 Apr 15. Cancer Chemother Pharmacol. 2022. PMID: 35426526
-
Pharmacokinetics of gemcitabine and metabolites in a patient with double-sided nephrectomy: a case report and review of the literature.Oncologist. 2009 Sep;14(9):944-8. doi: 10.1634/theoncologist.2009-0111. Epub 2009 Sep 2. Oncologist. 2009. PMID: 19726456 Review.
Cited by
-
Equilibrative nucleoside transporter 1 genotype, cytidine deaminase activity and age predict gemcitabine plasma clearance in patients with solid tumours.Br J Clin Pharmacol. 2011 Mar;71(3):437-44. doi: 10.1111/j.1365-2125.2010.03838.x. Br J Clin Pharmacol. 2011. PMID: 21284703 Free PMC article.
-
Sex differences in the pharmacokinetics of anticancer drugs: a systematic review.ESMO Open. 2024 Dec;9(12):104002. doi: 10.1016/j.esmoop.2024.104002. Epub 2024 Dec 10. ESMO Open. 2024. PMID: 39662226 Free PMC article.
-
A naïve pooled data approach for extrapolation of Phase 0 microdose trials to therapeutic dosing regimens.Clin Transl Sci. 2023 Feb;16(2):258-268. doi: 10.1111/cts.13446. Epub 2022 Nov 23. Clin Transl Sci. 2023. PMID: 36419385 Free PMC article.
-
A Phase I study of pazopanib in combination with gemcitabine in patients with advanced solid tumors.Cancer Chemother Pharmacol. 2013 Jan;71(1):93-101. doi: 10.1007/s00280-012-1982-z. Epub 2012 Oct 11. Cancer Chemother Pharmacol. 2013. PMID: 23064954 Free PMC article. Clinical Trial.
-
Severe acute toxicity following gemcitabine administration: A report of four cases with cytidine deaminase polymorphisms evaluation.Oncol Lett. 2018 Feb;15(2):1912-1916. doi: 10.3892/ol.2017.7473. Epub 2017 Nov 22. Oncol Lett. 2018. PMID: 29434889 Free PMC article.
References
-
- Natale R. A ten-year review of progress in the treatment of non-small-cell lung cancer with gemcitabine. Lung Cancer. 2005;50:S2–4. - PubMed
-
- El-Rayes BF, Philip PA. A review of systemic therapy for advanced pancreatic cancer. Clin Adv Hematol Oncol. 2003;1:430–4. - PubMed
-
- Heinemann V. Gemcitabine in metastatic breast cancer. Expert Rev Anticancer Ther. 2005;5:429–43. - PubMed
-
- Faivre S, Le Chevalier T, Monnerat C, Lokiec F, Novello S, Taieb J, Pautier P, Lhomme C, Ruffie P, Kayitalire L, Armand JP, Raymond E. Phase I–II and pharmacokinetic study of gemcitabine combined with oxaliplatin in patients with advanced non-small-cell lung cancer and ovarian carcinoma. Ann Oncol. 2002;13:1479–89. - PubMed
-
- Venook AP, Egorin MJ, Rosner GL, Hollis D, Mani S, Hawkins M, Byrd J, Hohl R, Budman D, Meropol NJ, Ratain MJ. Phase I and pharmacokinetic trial of gemcitabine in patients with hepatic or renal dysfunction. Cancer and Leukemia Group B 9565. J Clin Oncol. 2000;18:2780–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

