Control of gag-pol gene expression in the Candida albicans retrotransposon Tca2
- PMID: 17961216
- PMCID: PMC2194720
- DOI: 10.1186/1471-2199-8-94
Control of gag-pol gene expression in the Candida albicans retrotransposon Tca2
Abstract
Background: In the C. albicans retrotransposon Tca2, the gag and pol ORFs are separated by a UGA stop codon, 3' of which is a potential RNA pseudoknot. It is unclear how the Tca2 gag UGA codon is bypassed to allow pol expression. However, in other retroelements, translational readthrough of the gag stop codon can be directed by its flanking sequence, including a 3' pseudoknot.
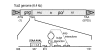
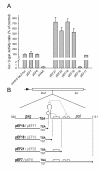
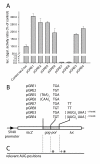
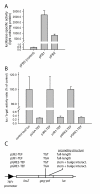
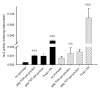
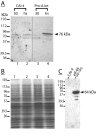
Results: The hypothesis was tested that in Tca2, gag stop codon flanking sequences direct translational readthrough and synthesis of a gag-pol fusion protein. Sequence from the Tca2 gag-UGA-pol junction (300 nt) was inserted between fused lacZ and luciferase (luc) genes in a Saccharomyces cerevisiae dual reporter construct. Although downstream of UGA, luc was expressed, but its expression was unaffected by inserting additional stop codons at the 3' end of lacZ. Luc expression was instead being driven by a previously unknown minor promoter activity within the gag-pol junction region. Evidence together indicated that junction sequence alone cannot direct UGA readthrough. Using reporter genes in C. albicans, the activities of this gag-pol junction promoter and the Tca2 long terminal repeat (LTR) promoter were compared. Of the two promoters, only the LTR promoter was induced by heat-shock, which also triggers retrotransposition. Tca2 pol protein, epitope-tagged in C. albicans to allow detection, was also heat-shock induced, indicating that pol proteins were expressed from a gag-UGA-pol RNA.
Conclusion: This is the first demonstration that the LTR promoter directs Tca2 pol protein expression, and that pol proteins are translated from a gag-pol RNA, which thus requires a mechanism for stop codon bypass. However, in contrast to most other retroelement and viral readthrough signals, immediate gag UGA-flanking sequences were insufficient to direct stop readthrough in S. cerevisiae, indicating non-canonical mechanisms direct gag UGA bypass in Tca2.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

