Solution NMR of membrane proteins in bilayer mimics: small is beautiful, but sometimes bigger is better
- PMID: 17961504
- PMCID: PMC2239318
- DOI: 10.1016/j.bbamem.2007.09.006
Solution NMR of membrane proteins in bilayer mimics: small is beautiful, but sometimes bigger is better
Abstract
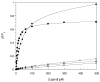
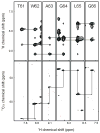
Considerable progress has been made recently on solution NMR studies of multi-transmembrane helix membrane protein systems of increasing size. Careful correlation of structure with function has validated the physiological relevance of these studies in detergent micelles. However, larger micelle and bicelle systems are sometimes required to stabilize the active forms of dynamic membrane proteins, such as the bacterial small multidrug resistance transporters. Even in these systems with aggregate molecular weights well over 100 kDa, solution NMR structural studies are feasible-but challenging.
Figures


References
-
- Hwang PM, Kay LE. Solution structure and dynamics of integral membrane proteins by NMR: a case study involving the enzyme PagP. Methods Enzymol. 2005;394:335–350. - PubMed
-
- Sanders CR, Sönnichsen F. Solution NMR of membrane proteins: practice and challenges. Magn Reson Chem. 2006;44:S24–40. - PubMed
-
- Tian C, Karra MD, Ellis CD, Jacob J, Oxenoid K, Sönnichsen F, Sanders CR. Membrane protein preparation for TROSY NMR screening. Methods Enzymol. 2005;394:321–334. - PubMed
-
- Tamm LK, Liang B. NMR of membrane proteins in solution. Prog Nucl Mag Res Sp. 2006;48:201–210.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

