Zebrafish: a model system for the study of eye genetics
- PMID: 17962065
- PMCID: PMC2271117
- DOI: 10.1016/j.preteyeres.2007.08.002
Zebrafish: a model system for the study of eye genetics
Abstract
Over the last decade, the use of the zebrafish as a genetic model has moved beyond the proof-of-concept for the analysis of vertebrate embryonic development to demonstrated utility as a mainstream model organism for the understanding of human disease. The initial identification of a variety of zebrafish mutations affecting the eye and retina, and the subsequent cloning of mutated genes have revealed cellular, molecular and physiological processes fundamental to visual system development. With the increasing development of genetic manipulations, sophisticated techniques for phenotypic characterization, behavioral approaches and screening strategies, the identification of novel genes or novel gene functions will have important implications for our understanding of human eye diseases, pathogenesis, and treatment.
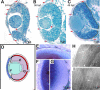
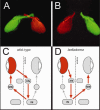
Figures




References
-
- Ahmad I, Dooley CM, Polk DL. Delta-1 is a regulator of neurogenesis in the vertebrate retina. Dev. Biol. 1997;185:92–103. - PubMed
-
- Ahmad I, Tang L, Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem. Biophys. Res. Commun. 2000;270:517–521. - PubMed
-
- Allende ML, Amsterdam A, Becker T, et al. Insertional mutagenesis in zebrafish Identifies two novel genes, pescadillo and dead eye, essential for embryonic development. Genes. Dev. 1996;10:3141–55. - PubMed
-
- Amsterdam A, Hopkins N. Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends Genet. 2006;22:473–478. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

