Modulation of glycine potency in rat recombinant NMDA receptors containing chimeric NR2A/2D subunits expressed in Xenopus laevis oocytes
- PMID: 17962328
- PMCID: PMC2375544
- DOI: 10.1113/jphysiol.2007.143172
Modulation of glycine potency in rat recombinant NMDA receptors containing chimeric NR2A/2D subunits expressed in Xenopus laevis oocytes
Abstract
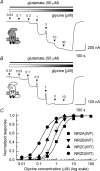
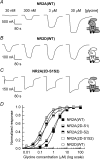
Heteromeric NMDARs are composed of coagonist glycine-binding NR1 subunits and glutamate-binding NR2 subunits. The majority of functional NMDARs in the mammalian central nervous system (CNS) contain two NR1 subunits and two NR2 subunits of which there are four types (A-D). We show that the potency of a variety of endogenous and synthetic glycine-site coagonists varies between recombinant NMDARs such that the highest potency is seen at NR2D-containing and the lowest at NR2A-containing NMDARs. This heterogeneity is specified by the particular NR2 subunit within the NMDAR complex since the glycine-binding NR1 subunit is common to all NMDARs investigated. To identify the molecular determinants responsible for this heterogeneity, we generated chimeric NR2A/2D subunits where we exchanged the S1 and S2 regions that form the ligand-binding domains and coexpressed these with NR1 subunits in Xenopus laevis oocytes. Glycine concentration-response curves for NMDARs containing NR2A subunits including the NR2D S1 region gave mean glycine EC(50) values similar to NR2A(WT)-containing NMDARs. However, receptors containing NR2A subunits including the NR2D S2 region or both NR2D S1 and S2 regions gave glycine potencies similar to those seen in NR2D(WT)-containing NMDARs. In particular, two residues in the S2 region of the NR2A subunit (Lys719 and Tyr735) when mutated to the corresponding residues found in the NR2D subunit influence glycine potency. We conclude that the variation in glycine potency is caused by interactions between the NR1 and NR2 ligand-binding domains that occur following agonist binding and which may be involved in the initial conformation changes that determine channel gating.
Figures








References
-
- Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nat Neurosci. 2003;6:144–152. - PubMed
-
- Baron BM, Harrison BL, Miller FP, McDonald IA, Salituro FG, Schmidt CJ, Sorensen SM, White HS, Palfreyman MG. Activity of 5,7-dichlorokynurenic acid, a potent antagonist at the N-methyl-D-aspartate receptor-associated glycine binding site. Mol Pharmacol. 1990;38:554–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

