Astrocytes play a critical role in transient heterosynaptic depression in the rat hippocampal CA1 region
- PMID: 17962333
- PMCID: PMC2375509
- DOI: 10.1113/jphysiol.2007.142737
Astrocytes play a critical role in transient heterosynaptic depression in the rat hippocampal CA1 region
Abstract
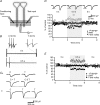
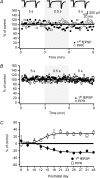
Active synapses can reduce the probability of transmitter release at neighbouring synapses. Depending on whether such heterosynaptic depression is mediated by intersynaptic diffusion of transmitter or by release of gliotransmitters, astrocytes should either hinder or promote the heterosynaptic depression. In the present study we have examined the developmental profile and astrocytic involvement in a transient heterosynaptic depression (tHeSD) in the CA1 region of the rat hippocampal slice preparation. A short stimulus burst (3 impulses at 50 Hz) to one group of synapses elicited a depression of the field EPSP evoked in another group of synapses that amounted to about 25% 0.5 s after the conditioning burst. This tHeSD was associated with an increase in the paired-pulse ratio of about 30%. The tHeSD was not present in slices from rats younger than 10 postnatal days and developed towards the adult magnitude between postnatal days 10 and 20. The tHeSD was totally prevented by the glia-specific toxin fluoroacetate (FAC), by carbenoxolone, a general blocker of connexin-based channels, and by endothelin, an endogenous peptide that has been shown to block astrocytic connexin-based channels. Antagonists to GABA(B) receptors and group II/III metabotropic glutamate receptors (mGluRs) abolished the tHeSD whereas antagonists to NMDA- and adenosine A1 receptors, and to group I mGluRs, did not affect the tHeSD. These results suggest that the tHeSD relies on GABA(B) receptors, group II/III mGluRs and on gliotransmitter release from functionally mature astrocytes.
Figures

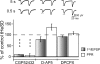
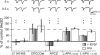

References
-
- Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol. 2005;15:542–548. - PubMed
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–2142. - PubMed
-
- Bains JS, Oliet SH. Glia: they make your memories stick! Trends Neurosci. 2007;30:417–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

