Cell proliferation and expression of cell cycle regulatory proteins that control the G1/S transition are age dependent and lobe specific in the Brown Norway rat model of prostatic hyperplasia
- PMID: 17962342
- PMCID: PMC2194603
- DOI: 10.1210/en.2007-1259
Cell proliferation and expression of cell cycle regulatory proteins that control the G1/S transition are age dependent and lobe specific in the Brown Norway rat model of prostatic hyperplasia
Abstract
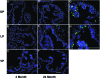
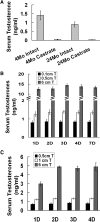
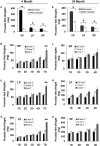
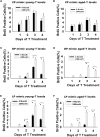
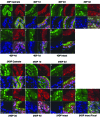
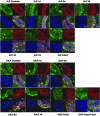
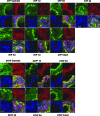
Age-dependent epithelial cell hyperplasia in the dorsal and lateral lobes of Brown Norway rats is analogous to benign prostatic hyperplasia in aging men. A major question is whether differential lobe-specific and age-dependent proliferation of cells, rather than cell survival, contributes to the hyperplasia. Although serum testosterone (T) levels decline in aged rats, active cell proliferation was detected as Ki67-positive cells in the dorsal and lateral lobes. We determined whether androgens differentially affect cell proliferation and cell-cycle regulatory proteins in the prostate lobes of young and aged rats. Castrated rats were treated with different doses of T to restore serum levels to those of intact young or aged rats. Rates of cell proliferation, measured by 5-bromodeoxyuridine labeling, peaked after 3-d T treatment in all lobes. 5-bromodeoxyuridine-labeling indices were higher in the dorsal and lateral lobes of aged than of young rats with equivalent serum T levels. No age-dependent difference was seen in the ventral lobe. Cell proliferation was marked by increased levels of cyclins D1 and E and cyclin-dependent kinases 4 and 6, decreased p27 and increased phosphorylation of Rb. Levels of cyclins D1 and E were higher in the dorsal and lateral lobes of intact and T-treated aged than young rats. Confocal immunofluorescent microscopy documented changes in cyclin-dependent kinase 4 and cyclin D1 subcellular localization. Cyclin D1 nuclear localization correlated with the time frame for cell proliferation. In conclusion, rates of cell proliferation and levels of cell-cycle regulatory proteins that control the G1/S transition exhibit lobe-specific and age-dependent differences in response to androgens.
Figures



Similar articles
-
Gene expression changes are age-dependent and lobe-specific in the brown Norway rat model of prostatic hyperplasia.Prostate. 2009 Jun 1;69(8):838-50. doi: 10.1002/pros.20935. Prostate. 2009. PMID: 19204916 Free PMC article.
-
Increased androgen receptor expression correlates with development of age-dependent, lobe-specific spontaneous hyperplasia of the brown Norway rat prostate.Endocrinology. 2001 Sep;142(9):4066-75. doi: 10.1210/endo.142.9.8376. Endocrinology. 2001. PMID: 11517186
-
Castration-induced apoptotic cell death in the Brown Norway rat prostate decreases as a function of age.Endocrinology. 2000 Feb;141(2):821-32. doi: 10.1210/endo.141.2.7339. Endocrinology. 2000. PMID: 10650965
-
Age-dependent and lobe-specific spontaneous hyperplasia in the brown Norway rat prostate.Biol Reprod. 1998 Nov;59(5):1163-70. doi: 10.1095/biolreprod59.5.1163. Biol Reprod. 1998. PMID: 9780323
-
Location, location, location: the role of cyclin D1 nuclear localization in cancer.J Cell Biochem. 2005 Dec 1;96(5):906-13. doi: 10.1002/jcb.20613. J Cell Biochem. 2005. PMID: 16163738 Review.
Cited by
-
Androgen action in prostate function and disease.Am J Clin Exp Urol. 2018 Apr 1;6(2):62-77. eCollection 2018. Am J Clin Exp Urol. 2018. PMID: 29666834 Free PMC article. Review.
-
Gene expression changes are age-dependent and lobe-specific in the brown Norway rat model of prostatic hyperplasia.Prostate. 2009 Jun 1;69(8):838-50. doi: 10.1002/pros.20935. Prostate. 2009. PMID: 19204916 Free PMC article.
-
Reduction of insulin signalling pathway IRS-1/IRS-2/AKT/mTOR and decrease of epithelial cell proliferation in the prostate of glucocorticoid-treated rats.Int J Exp Pathol. 2012 Jun;93(3):188-95. doi: 10.1111/j.1365-2613.2012.00817.x. Int J Exp Pathol. 2012. PMID: 22583132 Free PMC article.
-
Proliferation and apoptotic rates and increased frequency of p63-positive cells in the prostate acinar epithelium of alloxan-induced diabetic rats.Int J Exp Pathol. 2010 Apr;91(2):144-54. doi: 10.1111/j.1365-2613.2009.00696.x. Epub 2009 Dec 22. Int J Exp Pathol. 2010. PMID: 20041964 Free PMC article.
References
-
- Price D 1963 Comparative aspects of development and structure in the prostate. Natl Cancer Inst Monogr 12:351–369 - PubMed
-
- Banerjee PP, Banerjee S, Lai JM, Strandberg, JD, Zirkin BR and Brown TR 1998 Age-dependent and lobe-specific spontaneous hyperplasia in the Brown Norway rat prostate. Biol Reprod 59:1163–1170 - PubMed
-
- Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, Bi BY, Chatterjee B 1999 Regulation of androgen action. Vitam Horm 55:309–352 - PubMed
-
- Isaacs JT 2005 New strategies for the medical treatment of prostate cancer. BJU Int 96(Suppl 2):35–40 - PubMed
-
- Cuellar DC, Kyprianou N 2001 Future concepts in the medical therapy of benign prostatic hyperplasia. Curr Opin Urol 11:27–33 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

