Protein aggregation processes: In search of the mechanism
- PMID: 17962399
- PMCID: PMC2211696
- DOI: 10.1110/ps.073164107
Protein aggregation processes: In search of the mechanism
Abstract
Amyloid formation typically follows a time course in which there is a long lag period followed by a rapid formation of fibrils. In this review, I show that the standard mechanisms of polymerization need to be expanded to consider that the monomeric proteins/peptides involved in amyloid formation are intrinsically disordered and exist as an ensemble of disordered-collapsed states. The review focuses primarily on events which occur in the long lag period defining these as protein folding issues, coupled with formation of oligomers. Experimental methods to explore folding and oligomerization issues over a wide range of protein concentrations using primarily fluorescence and 19F-NMR methods are discussed.
Figures
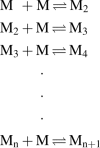


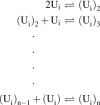
References
-
- Balbach J.J., Petkova, A.T., Oyler, N.A., Antzutkin, O.N., Gordon, D.J., Meredith, S.C., and Tycko, R. 2002. Supramolecular structure in full-length Alzheimer's β-amyloid fibrils: Evidence for a parallel β-sheet organization from solid-state nuclear magnetic resonance. Biophys. J. 83: 1205–1216. - PMC - PubMed
-
- Bann J.G. and Frieden, C. 2004. Folding and domain–domain interactions of the chaperone PapD measured by 19F NMR. Biochemistry 43: 13775–13786. - PubMed
-
- Barshop B.A., Wrenn, R.F., and Frieden, C. 1983. Analysis of numerical methods for computer simulation of kinetic processes: Development of KINSIM—A flexible, portable system. Anal. Biochem. 130: 134–145. - PubMed
-
- Cannon M.J., Williams, A.D., Wetzel, R., and Myszka, D.G. 2004. Kinetic analysis of β-amyloid fibril elongation. Anal. Biochem. 328: 67–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

