Galpha Gbetagamma dissociation may be due to retraction of a buried lysine and disruption of an aromatic cluster by a GTP-sensing Arg Trp pair
- PMID: 17962409
- PMCID: PMC2211685
- DOI: 10.1110/ps.073098107
Galpha Gbetagamma dissociation may be due to retraction of a buried lysine and disruption of an aromatic cluster by a GTP-sensing Arg Trp pair
Abstract
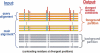
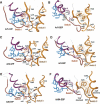
The heterotrimeric G protein alpha subunit (Galpha) functions as a molecular switch by cycling between inactive GDP-bound and active GTP-bound states. When bound to GDP, Galpha interacts with high affinity to a complex of the beta and gamma subunits (Gbetagamma), but when bound to GTP, Galpha dissociates from this complex to activate downstream signaling pathways. Galpha's state is communicated to other cellular components via conformational changes within its switch I and II regions. To identify key determinants of Galpha's function as a signaling pathway molecular switch, a Bayesian approach was used to infer the selective constraints that most distinguish Galpha and closely related Arf family GTPases from distantly related translational and metabolic GTPases. The strongest of these constraints are imposed on seven residues within or near the switch II region. Likewise, constraints imposed on Galpha but not on other, closely related molecular switches correspond to four nearby residues. These constraints are explained by a proposed mechanism for GTP-induced dissociation of Galpha from Gbetagamma where an Arg-Trp pair senses the presence of bound GTP leading to conformational retraction of a nearby lysine and to disruption of an aromatic cluster. Within a complex of Gialpha, Gibetagamma, and GDP, this lysine establishes greater surface contact with Gibeta than does any other residue in Gialpha, whereas the aromatic cluster packs against a highly conserved tryptophan in Gibeta that establishes greater surface contact with Gialpha than does any other residue in Gibeta. Other structural features associated with Galpha functional divergence further support the proposed mechanism.
Figures

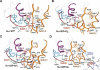
References
-
- Amor J.C., Harrison, D.H., Kahn, R.A., and Ringe, D. 1994. Structure of the human ADP-ribosylation factor 1 complexed with GDP. Nature 372: 704–708. - PubMed
-
- Atanassova A., Tempel, W., Dimov, S., Yaniw, D., Arrowsmith, C., Edwards, A., Sundstrom, M., Weigelt, J., Bochkarev, A., and Park, H. 2006. Structure of human Adp-ribosylation factor-like 10b (Arl10b). Structural Genomics Consortium, Toronto, Canada.
-
- Berghuis A.M., Lee, E., Raw, A.S., Gilman, A.G., and Sprang, S.R. 1996. Structure of the GDP–Pi complex of Gly203 → Ala giα1: A mimic of the ternary product complex of gα-catalyzed GTP hydrolysis. Structure 4: 1277–1290. - PubMed
-
- Burd C.G., Strochlic, T.I., and Gangi Setty, S.R. 2004. Arf-like GTPases: Not so Arf-like after all. Trends Cell Biol. 14: 687–694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

