Human G(salpha) mutant causes pseudohypoparathyroidism type Ia/neonatal diarrhea, a potential cell-specific role of the palmitoylation cycle
- PMID: 17962410
- PMCID: PMC2077272
- DOI: 10.1073/pnas.0708561104
Human G(salpha) mutant causes pseudohypoparathyroidism type Ia/neonatal diarrhea, a potential cell-specific role of the palmitoylation cycle
Abstract
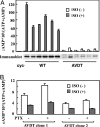
Pseudohypoparathyroidism type Ia (PHP-Ia) results from the loss of one allele of G(salpha), causing resistance to parathyroid hormone and other hormones that transduce signals via G(s). Most G(salpha)mutations cause the complete loss of protein expression, but some cause loss of function only, and these have provided valuable insights into the normal function of G proteins. Here we have analyzed a mutant G(salpha) (alphas-AVDT) harboring AVDT amino acid repeats within its GDP/GTP binding site, which was identified in unique patients with PHP-Ia accompanied by neonatal diarrhea. Biochemical and intact cell analyses showed that alphas-AVDT is unstable but constitutively active as a result of rapid GDP release and reduced GTP hydrolysis. This instability underlies the PHP-Ia phenotype. alphas-AVDT is predominantly localized in the cytosol, but in rat and mouse small intestine epithelial cells (IEC-6 and DIF-12 cells) alphas-AVDT was found to be localized predominantly in the membrane where adenylyl cyclase is present and constitutive increases in cAMP accumulation occur in parallel. The likely cause of this membrane localization is the inhibition of an activation-dependent decrease in alphas palmitoylation. Upon the overexpression of acyl-protein thioesterase 1, however, alphas-AVDT translocates from the membrane to the cytosol, and the constitutive accumulation of cAMP becomes attenuated. These results suggest that PHP-Ia results from the instability of alphas-AVDT and that the accompanying neonatal diarrhea may result from its enhanced constitutive activity in the intestine. Hence, palmitoylation may control the activity and localization of G(salpha) in a cell-specific manner.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Farfel Z, Bourne HR, Iiri T. N Engl J Med. 1999;340:1012–1020. - PubMed
-
- Spiegel AM, Weinstein LS. Annu Rev Med. 2004;55:27–39. - PubMed
-
- Aldred MA. J Pediatr Endocrinol Metab. 2006;19:S635–S640. - PubMed
-
- Lania AG, Mantovani G, Spada A. Nat Clin Pract Endocrinol Metab. 2006;2:681–693. - PubMed
-
- Iiri T, Farfel Z, Bourne HR. Nature. 1998;394:35–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

