Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma
- PMID: 17962455
- PMCID: PMC2874676
- DOI: 10.1167/iovs.07-0587
Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma
Abstract
Purpose: To investigate the potential of human corneal stromal stem cells to assume a keratocyte phenotype and to organize extracellular matrix (ECM) in vitro similar to corneal stromal tissue.
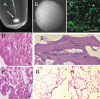
Methods: Human corneal stromal stem cells (hCSSC) were isolated as side population cells by flow cytometry. Cloned hCSSC were cultured as free-floating pellets in serum-free media for 3 weeks. Gene expression was examined using gene array, quantitative RT-PCR, immunostaining, and immunoblotting. Transmission electron microscopy showed collagen fibril size and alignment.
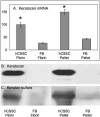
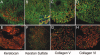
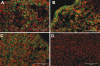
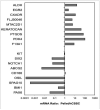
Results: Pellet cultures of hCSSC in serum-free media upregulated the expression of keratocyte-specific genes and secreted substantial ECM containing characteristic stromal components: keratocan, keratan sulfate, collagen I, collagen V, and collagen VI. Abundant connexin 43 and cadherin 11 in pellets demonstrated cell-cell junctions typical of keratocytes in vivo. Electron microscopy of the pellet cultures revealed abundant fibrillar collagen, some of which was aligned in parallel arrays similar to those of stromal lamellae. Gene array identified expression in pellets of several genes highly expressed by keratocytes. Transcripts for these keratocyte genes -- FLJ30046, KERA, ALDH3A1, CXADR, PTGDS, PDK4, MTAC2D1, F13A1 -- were increased by as much as 100-fold in pellets compared with hCSSC. Simultaneously, expression of stem cell genes BMI1, KIT, NOTCH1, SIX2, PAX6, ABCG2, SPAG10, and OSIL was reduced by a similar factor in pellets compared with hCSSC.
Conclusions: Scaffolding-free pellet culture of hCSSC induces keratocyte gene expression patterns in these cells and secretion of an organized stroma-like ECM. These cells offer a novel potential for corneal bioengineering.
Figures


Similar articles
-
The effect of growth factor supplementation on corneal stromal cell phenotype in vitro using a serum-free media.Exp Eye Res. 2016 Oct;151:26-37. doi: 10.1016/j.exer.2016.07.015. Epub 2016 Jul 22. Exp Eye Res. 2016. PMID: 27456135
-
Adipose-derived stem cells differentiate to keratocytes in vitro.Mol Vis. 2010 Dec 10;16:2680-9. Mol Vis. 2010. PMID: 21179234 Free PMC article.
-
Corneal stromal stem cells versus corneal fibroblasts in generating structurally appropriate corneal stromal tissue.Exp Eye Res. 2014 Mar;120:71-81. doi: 10.1016/j.exer.2014.01.005. Epub 2014 Jan 15. Exp Eye Res. 2014. PMID: 24440595 Free PMC article.
-
The molecular basis of corneal transparency.Exp Eye Res. 2010 Sep;91(3):326-35. doi: 10.1016/j.exer.2010.06.021. Epub 2010 Jul 3. Exp Eye Res. 2010. PMID: 20599432 Free PMC article. Review.
-
Keratocyte biology.Exp Eye Res. 2020 Jul;196:108062. doi: 10.1016/j.exer.2020.108062. Epub 2020 May 19. Exp Eye Res. 2020. PMID: 32442558 Review.
Cited by
-
Quantitative assessment of ultrastructure and light scatter in mouse corneal debridement wounds.Invest Ophthalmol Vis Sci. 2012 May 14;53(6):2786-95. doi: 10.1167/iovs.11-9305. Invest Ophthalmol Vis Sci. 2012. PMID: 22467580 Free PMC article.
-
Concise review: Stem cells in the corneal stroma.Stem Cells. 2012 Jun;30(6):1059-63. doi: 10.1002/stem.1100. Stem Cells. 2012. PMID: 22489057 Free PMC article. Review.
-
Cytoprotective Effects of Human Platelet Lysate during the Xeno-Free Culture of Human Donor Corneas.Int J Mol Sci. 2023 Feb 2;24(3):2882. doi: 10.3390/ijms24032882. Int J Mol Sci. 2023. PMID: 36769200 Free PMC article.
-
Keratocyte phenotype is enhanced in the absence of attachment to the substratum.Mol Vis. 2008 Feb 9;14:308-17. Mol Vis. 2008. PMID: 18334944 Free PMC article.
-
Stem Cells in the Cornea.Prog Mol Biol Transl Sci. 2015;134:25-41. doi: 10.1016/bs.pmbts.2015.04.002. Epub 2015 May 27. Prog Mol Biol Transl Sci. 2015. PMID: 26310147 Free PMC article. Review.
References
-
- Funderburgh JL. Corneal proteoglycans. In: Iozzo R, editor. Proteoglycans: Structure, Biology, and Molecular Interactions. Marcel Dekker, Inc; New York: 2000. pp. 237–273.
-
- Long CJ, Roth MR, Tasheva ES, et al. Fibroblast growth factor-2 promotes keratan sulfate proteoglycan expression by keratocytes in vitro. J Biol Chem. 2000;275:13918–13923. - PubMed
-
- Edward DP, Yue BY, Sugar J, et al. Heterogeneity in macular corneal dystrophy. Arch Ophthalmol. 1988;106:1579–1583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

