Identifying the role of specific motifs in the lens fiber cell specific intermediate filament phakosin
- PMID: 17962466
- PMCID: PMC2909742
- DOI: 10.1167/iovs.07-0647
Identifying the role of specific motifs in the lens fiber cell specific intermediate filament phakosin
Abstract
Purpose: Phakosin and filensin are lens fiber cell-specific intermediate filament (IF) proteins. Unlike every other cytoplasmic IF protein, they assemble into a beaded filament (BF) rather than an IF. Why the lens fiber cell requires two unique IF proteins and why and how they assemble into a structure other than an IF are unknown. In this report we test specific motifs/domains in phakosin to identify changes that that have adapted phakosin to lens-specific structure and function.
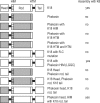
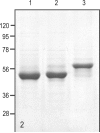
Methods: Phakosin shows the highest level of sequence identity to K18, whose natural assembly partner is K8. We therefore exchanged conserved keratin motifs between phakosin and K18 to determine whether phakosin's divergent motifs could redirect the assembly of chimeric K18 and K8. Modified proteins were bacterially expressed and purified. Assembly competence was assessed by electron microscopy.
Results: Substitution of the phakosin helix initiation motif (HIM) into K18 does not alter assembly with K8, establishing that the radical divergence in phakosin HIM is not by itself the mechanism by which IF assembly is redirected to BF assembly. Unexpectedly, K18 bearing phakosin HIM resulted in normal IF assembly, despite the presence of an otherwise disease-causing R-C substitution, and two helix-disrupting glycines. This disproves the widely held belief that mutation of the R is catastrophic to IF assembly. Additional data are presented that suggest normal IF assembly is dependent on sequence-specific interactions between the IF head domain and the HIM.
Conclusions: In the lens fiber cell, two members of the IF family have evolved to produce BFs instead of IFs, a change that presumably adapts the IF to a fiber cell-specific function. The authors establish here that the most striking divergence seen in phakosin is not, as hypothesized, the cause of this altered assembly outcome. The authors further establish that the HIM of IFs is far more tolerant of mutations, such as those that cause some corneal dystrophies and Alexander disease, than previously hypothesized and that normal assembly involves sequence-specific interactions between the head domain and the HIM.
Figures





Similar articles
-
Resisting the effects of aging: a function for the fiber cell beaded filament.Invest Ophthalmol Vis Sci. 2008 Mar;49(3):1030-6. doi: 10.1167/iovs.07-1149. Invest Ophthalmol Vis Sci. 2008. PMID: 18326727 Free PMC article.
-
Targeted deletion of the lens fiber cell-specific intermediate filament protein filensin.Invest Ophthalmol Vis Sci. 2003 Dec;44(12):5252-8. doi: 10.1167/iovs.03-0224. Invest Ophthalmol Vis Sci. 2003. PMID: 14638724
-
Primary sequence, secondary structure, gene structure, and assembly properties suggests that the lens-specific cytoskeletal protein filensin represents a novel class of intermediate filament protein.Exp Eye Res. 1998 May;66(5):625-44. doi: 10.1006/exer.1998.0478. Exp Eye Res. 1998. PMID: 9628810
-
The beaded intermediate filaments and their potential functions in eye lens.Bioessays. 1994 Jun;16(6):413-8. doi: 10.1002/bies.950160609. Bioessays. 1994. PMID: 8080431 Review.
-
Insights into the beaded filament of the eye lens.Exp Cell Res. 2007 Jun 10;313(10):2180-8. doi: 10.1016/j.yexcr.2007.04.005. Epub 2007 Apr 6. Exp Cell Res. 2007. PMID: 17490642 Free PMC article. Review.
Cited by
-
Effects of truncations in the N- and C-terminal domains of filensin on filament formation with phakinin in cell-free conditions and cultured cells.FEBS Open Bio. 2023 Nov;13(11):1990-2004. doi: 10.1002/2211-5463.13700. Epub 2023 Aug 30. FEBS Open Bio. 2023. PMID: 37615966 Free PMC article.
-
Effect of the Rho-Kinase/ROCK Signaling Pathway on Cytoskeleton Components.Genes (Basel). 2023 Jan 20;14(2):272. doi: 10.3390/genes14020272. Genes (Basel). 2023. PMID: 36833199 Free PMC article. Review.
-
Lens intermediate filaments.Exp Eye Res. 2009 Feb;88(2):165-72. doi: 10.1016/j.exer.2008.11.007. Epub 2008 Nov 24. Exp Eye Res. 2009. PMID: 19071112 Free PMC article. Review.
References
-
- Herrmann H, Aebi U. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular scaffolds. Annu Rev Biochem. 2004;73:749–789. - PubMed
-
- Parry DAD, Steinert PM. Intermediate Filament Structure. R. G. Landes Company; Austin, TX: 1995.
-
- Steinert PM, Parry DA. Intermediate filaments: conformity and diversity of expression and structure. Annu Rev Cell Biol. 1985;1:41–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

