Extravasations and emigration of neutrophils to the inflammatory site depend on the interaction of immune-complex with Fcgamma receptors and can be effectively blocked by decoy Fcgamma receptors
- PMID: 17962513
- PMCID: PMC2200857
- DOI: 10.1182/blood-2007-04-085944
Extravasations and emigration of neutrophils to the inflammatory site depend on the interaction of immune-complex with Fcgamma receptors and can be effectively blocked by decoy Fcgamma receptors
Abstract
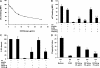
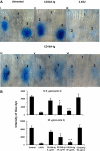
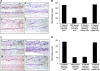
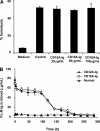
Extravasation and emigration of neutrophils to the site of inflammation are essential early steps in the initiation of many antibody-mediated autoimmune diseases. The Fc domains of cell bound autoantibodies or immune-complexes (IC) are capable of triggering the neutrophil emigration via complement and FcgammaRs-mediated mechanisms. To define the clinical relevance and the relative contribution of these 2 pathways in IC-mediated neutrophil emigration, we have neutralized the FcgammaR-binding activity of IC with a recombinant dimeric Fc receptor, CD16A-Ig, and investigated the early events of IC-induced inflammation in mice. Systemic administration of purified CD16A-Ig blocked IC-induced inflammation, mast- cell degranulation, and extravasation of neutrophils in a reversed Arthus reaction. Although the binding of CD16A-Ig to IC did not alter the complement-activating properties of IC, no evidence for complement-dependent neutrophil emigration was observed. These results suggest that interaction of IC with cells expressing FcgammaRs at the inflammatory site results in the secretion of chemoattractants, which mediate complement-independent emigration of neutrophils in this cutaneous acute inflammation model. Furthermore, blocking the interaction of IC to FcgammaRs expressed on inflammatory cells by administering high-avidity Fc fusion dimers of low-affinity FcgammaRs is an effective way of preventing IC-induced acute inflammation in autoimmune diseases.
Figures


Similar articles
-
Hydrodynamic delivery of plasmid DNA encoding human FcγR-Ig dimers blocks immune-complex mediated inflammation in mice.Gene Ther. 2012 Sep;19(9):877-85. doi: 10.1038/gt.2011.175. Epub 2011 Nov 24. Gene Ther. 2012. PMID: 22113315 Free PMC article.
-
The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases.Immunobiology. 2012 Nov;217(11):1067-79. doi: 10.1016/j.imbio.2012.07.015. Immunobiology. 2012. PMID: 22964232 Review.
-
rIgG1 Fc Hexamer Inhibits Antibody-Mediated Autoimmune Disease via Effects on Complement and FcγRs.J Immunol. 2018 Apr 15;200(8):2542-2553. doi: 10.4049/jimmunol.1701171. Epub 2018 Mar 12. J Immunol. 2018. PMID: 29531170 Free PMC article.
-
Requirement for Vav proteins in post-recruitment neutrophil cytotoxicity in IgG but not complement C3-dependent injury.J Immunol. 2008 May 1;180(9):6279-87. doi: 10.4049/jimmunol.180.9.6279. J Immunol. 2008. PMID: 18424751
-
Fc-gamma receptors: Attractive targets for autoimmune drug discovery searching for intelligent therapeutic designs.Autoimmun Rev. 2016 Nov;15(11):1081-1088. doi: 10.1016/j.autrev.2016.07.035. Epub 2016 Aug 1. Autoimmun Rev. 2016. PMID: 27491569 Review.
Cited by
-
Hydrodynamic delivery of plasmid DNA encoding human FcγR-Ig dimers blocks immune-complex mediated inflammation in mice.Gene Ther. 2012 Sep;19(9):877-85. doi: 10.1038/gt.2011.175. Epub 2011 Nov 24. Gene Ther. 2012. PMID: 22113315 Free PMC article.
-
Inhibition of zymosan-induced kidney dysfunction by tyrphostin AG-490.J Inflamm (Lond). 2009 May 5;6:13. doi: 10.1186/1476-9255-6-13. J Inflamm (Lond). 2009. PMID: 19416544 Free PMC article.
-
Immune Complex-Induced, Nitric Oxide-Mediated Vascular Endothelial Cell Death by Phagocytes Is Prevented with Decoy FcγReceptors.PLoS One. 2016 Apr 21;11(4):e0153620. doi: 10.1371/journal.pone.0153620. eCollection 2016. PLoS One. 2016. PMID: 27101012 Free PMC article.
-
Differential role of lipocalin 2 during immune complex-mediated acute and chronic inflammation in mice.Arthritis Rheum. 2013 Apr;65(4):1064-73. doi: 10.1002/art.37840. Arthritis Rheum. 2013. PMID: 23280250 Free PMC article.
-
Hsp60 and Hsp10 increase in colon mucosa of Crohn’s disease and ulcerative colitis.Cell Stress Chaperones. 2010 Nov;15(6):877-84. doi: 10.1007/s12192-010-0196-8. Cell Stress Chaperones. 2010. PMID: 20390473 Free PMC article.
References
-
- Centers for Disease Control. The most frequent causes of disability among Americans aged 18 years or older. MMWR. 2001;50:120–125. - PubMed
-
- Schwartz RS. Autoimmunity and autoimmune diseases. In: Paul WE, editor. Fundamental Immunology. New York: Raven Press, Ltd; 1993. pp. 1033–1097.
-
- Abbas AK, Lichtman AH, Pober JS. Cellular and Molecular Immunology. Philadelphia PA: W.B. Saunders; 1997. Immune-mediated tissue injury and disease. pp. 423–438.
-
- Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. - PubMed
-
- Müller-Eberhard HJ. Molecular organisation of the complement system. Annu Rev Biochem. 1988;57:321–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

