IFN-gamma production during initial infection determines the outcome of reinfection with respiratory syncytial virus
- PMID: 17962634
- PMCID: PMC2204078
- DOI: 10.1164/rccm.200612-1890OC
IFN-gamma production during initial infection determines the outcome of reinfection with respiratory syncytial virus
Abstract
Rationale: Severe respiratory syncytial virus (RSV) bronchiolitis has been associated with deficient IFN-gamma production in humans, but the role of this cytokine in determining the outcome of reinfection is unknown.
Objectives: To define the role of IFN-gamma in the development of RSV-mediated airway hyperresponsiveness (AHR) and lung histopathology in mice.
Methods: Wild-type (WT) and IFN-gamma knockout mice were infected with RSV in the newborn or weaning stages and reinfected 5 weeks later. Airway responses were assessed on Day 6 after the primary or secondary infection.
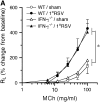
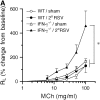
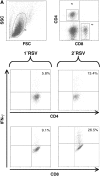
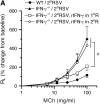
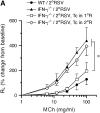
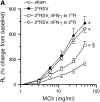
Measurements and main results: Both WT and IFN-gamma knockout mice developed similar levels of AHR and airway inflammation after primary infection. After reinfection, IFN-gamma knockout mice, but not WT mice, developed AHR, airway eosinophilia, and mucus hyperproduction. Intranasal administration of IFN-gamma during primary infection but not during reinfection prevented the development of these altered airway responses on reinfection in IFN-gamma knockout mice. Adoptive transfer of WT T cells into IFN-gamma knockout mice before primary infection restored IFN-gamma production in the lungs and prevented the development of altered airway responses on reinfection. Treatment of mice with IFN-gamma during primary neonatal infection prevented the enhancement of AHR and the development of airway eosinophilia and mucus hyperproduction on reinfection.
Conclusions: IFN-gamma production during primary RSV infection is critical to the development of protection against AHR and lung histopathology on reinfection. Provision of IFN-gamma during primary infection in infancy may be a potential therapeutic approach to alter the course of RSV-mediated long-term sequelae.
Figures




















References
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986;140:543–546. - PubMed
-
- Hall CB. Respiratory syncytial virus: a continuing culprit and conundrum. J Pediatr 1999;135:2–7. - PubMed
-
- Falsey AR, Walsh EE. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J Infect Dis 1998;177:463–466. - PubMed
-
- Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;354:541–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

