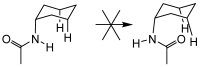Bile acid scaffolds in supramolecular chemistry: the interplay of design and synthesis
- PMID: 17962729
- PMCID: PMC6149150
- DOI: 10.3390/12082106
Bile acid scaffolds in supramolecular chemistry: the interplay of design and synthesis
Abstract
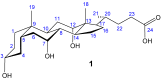
Since early work in the 1980s, the bile acids have become well established as building blocks for supramolecular chemistry. The author's laboratory has specialised in converting cholic acid, the archetypal bile acid, into macrocyclic and acyclic receptors for anions and carbohydrates. This review highlights the synthetic aspects of this work, especially the use of modern synthetic methodology to perform less obvious structural transformations.
Figures
Similar articles
-
Chemical and metabolic transformations of selected bile acids.Eur J Drug Metab Pharmacokinet. 2006 Jul-Sep;31(3):179-235. doi: 10.1007/BF03190713. Eur J Drug Metab Pharmacokinet. 2006. PMID: 17136861 Review.
-
Improved synthesis and characterization of bile acid esters: Organogelation and supramolecular properties.Steroids. 2025 Feb;214:109560. doi: 10.1016/j.steroids.2025.109560. Epub 2025 Jan 9. Steroids. 2025. PMID: 39793913
-
Cholic acid in guinea pig bile.Proc Soc Exp Biol Med. 1960 Nov;105:337-9. doi: 10.3181/00379727-105-26103. Proc Soc Exp Biol Med. 1960. PMID: 13734368 No abstract available.
-
Synthesis, aggregation behavior and cholesterol solubilization studies of 16-epi-pythocholic acid (3 alpha,12 alpha,16 beta-trihydroxy-5 beta-cholan-24-oic acid).Steroids. 2010 Jul;75(7):506-12. doi: 10.1016/j.steroids.2010.03.007. Epub 2010 Mar 30. Steroids. 2010. PMID: 20359489
-
Recent methods for diversification of bile acids and related steroids towards supramolecular.Steroids. 2019 Nov;151:108442. doi: 10.1016/j.steroids.2019.108442. Epub 2019 Jul 19. Steroids. 2019. PMID: 31326451 Review.
Cited by
-
Regiochemical Effects on the Carbohydrate Binding and Selectivity of Flexible Synthetic Carbohydrate Receptors with Indole and Quinoline Heterocyclic Groups.European J Org Chem. 2021 Oct 7;2021(37):5262-5274. doi: 10.1002/ejoc.202100763. Epub 2021 Sep 12. European J Org Chem. 2021. PMID: 35694139 Free PMC article.
-
Pd- and Cu-catalyzed approaches in the syntheses of new cholane aminoanthraquinone pincer-like ligands.Beilstein J Org Chem. 2017 Mar 20;13:564-570. doi: 10.3762/bjoc.13.55. eCollection 2017. Beilstein J Org Chem. 2017. PMID: 28405236 Free PMC article.
-
Bile acids: chemistry, physiology, and pathophysiology.World J Gastroenterol. 2009 Feb 21;15(7):804-16. doi: 10.3748/wjg.15.804. World J Gastroenterol. 2009. PMID: 19230041 Free PMC article. Review.
-
Bis[3α,7α,12α-tris-(4-nitro-benzo-yloxy)-5β-cholan-24-yl] disulfide-ethyl acetate-n-hexane (4/4/1).Acta Crystallogr Sect E Struct Rep Online. 2010 Dec 11;67(Pt 1):o74-5. doi: 10.1107/S1600536810050385. Acta Crystallogr Sect E Struct Rep Online. 2010. PMID: 21522786 Free PMC article.
-
Role of the intestinal bile acid transporters in bile acid and drug disposition.Handb Exp Pharmacol. 2011;(201):169-203. doi: 10.1007/978-3-642-14541-4_4. Handb Exp Pharmacol. 2011. PMID: 21103970 Free PMC article. Review.
References
-
- McKenna J., McKenna J. M., Thornthwaite D. W. Bis-steroids as potential enzyme models: Perylene solubilisation and dye spectral changes with aqueous solutions of some derivatives of conessine and cholic acid. J. Chem. Soc., Chem. Commun. 1977:809–811. doi: 10.1039/c39770000809. - DOI
-
- Davis A. P. Cholaphanes et al.; Steroids as structural components in molecular engineering. Chem. Soc. Rev. 1993;22:243–253.
- Davis A. P., Bonar-Law R. P., Sanders J. K. M. In: Comprehensive Supramolecular Chemistry. Murakami Y., editor. Vol. 4. Pergamon; Oxford: 1996. pp. 257–286. (Supramolecular Reactivity and Transport: Bioorganic Systems)
- Li Y. X., Dias J. R. Dimeric and oligomeric steroids. Chem. Rev. 1997;97:283–304. - PubMed
- Tamminen J., Kolehmainen E. Bile acids as building blocks of supramolecular hosts. Molecules. 2001;6:21–46.
- Virtanen E., Kolehmainen E. Use of bile acids in pharmacological and supramolecular applications. Eur. J. Org. Chem. 2004:3385–3399.
-
-
We estimate that at least 40 groups have contributed to the area.
-
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources