Flavonoids: hemisynthesis, reactivity, characterization and free radical scavenging activity
- PMID: 17962740
- PMCID: PMC6149145
- DOI: 10.3390/12092228
Flavonoids: hemisynthesis, reactivity, characterization and free radical scavenging activity
Abstract
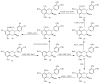
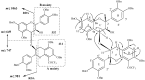
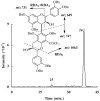
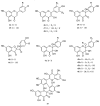
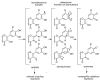
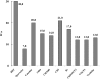
Phenolic compounds form one of the main classes of secondary metabolites. They display a large range of structures and they are responsible for the major organoleptic characteristics of plant-derived-foods and beverages, particularly color and taste properties and they also contribute to the nutritional qualities of fruits and vegetables. Phenolic compounds are also highly unstable compounds which undergo numerous enzymatic and chemical reactions during postharvest food storage and processing thus adding to the complexity of plant polyphenol composition. Among these compounds flavonoids constitute one of the most ubiquitous groups of all plant phenolics. Owing to their importance in food organoleptic properties and in human health, a better understanding of their structures, their reactivity and chemical properties in addition to the mechanisms generating them appears essential to predict and control food quality. The purpose of this work is an overview of our findings concerning the hemisynthesis, the reactivity and the enzymatic oxidation of some flavonoids and shed light on the mechanisms involved in some of these processes and the structures of the resulting products. The free radical scavenging activity of some of the synthesized compounds is also presented and a structure-activity relationship is discussed. The first part of this review concerns the synthesis and structural characterization of modified monomeric flavanols. The use of these compounds as precursor for the preparation of natural and modified dimeric procyanidin derivatives was then explored through different coupling reactions. The full characterization of the synthesized compounds was achieved by concerted use of NMR and ESI-MS techniques. The free radical scavenging activity of some of the synthesized compounds was investigated. The second part of this review concerns the enzymatic oxidation of several flavonols by Trametes versicolor laccase. Most of the major oxidation products have been isolated as pure compounds and their structures unambiguously established through spectroscopic methods. Correlation between the structure of the oxidation product and the substitution pattern of the starting materials allows mechanistic features of this transformation to be elucidated.
Figures















Similar articles
-
Comparative analysis of molecular properties and reactions with oxidants for quercetin, catechin, and naringenin.Mol Cell Biochem. 2021 Dec;476(12):4287-4299. doi: 10.1007/s11010-021-04243-w. Epub 2021 Aug 18. Mol Cell Biochem. 2021. PMID: 34406575 Free PMC article.
-
Antioxidant and Free Radical Scavenging Activity of Phenolics from Bidens humilis.Planta Med. 2015 Aug;81(12-13):1056-64. doi: 10.1055/s-0035-1545928. Epub 2015 Apr 23. Planta Med. 2015. PMID: 25905594
-
Inhibition of hazardous compound formation in muscle foods by antioxidative phytophenols.Ann N Y Acad Sci. 2017 Jun;1398(1):37-46. doi: 10.1111/nyas.13368. Epub 2017 Jun 12. Ann N Y Acad Sci. 2017. PMID: 28605826 Review.
-
Radical-scavenging polyphenols: new strategies for their synthesis.J Pharm Pharmacol. 2007 Dec;59(12):1703-10. doi: 10.1211/jpp.59.12.0013. J Pharm Pharmacol. 2007. PMID: 18053333 Review.
-
Theoretical investigation of the effect of sugar substitution on the antioxidant properties of flavonoids.Free Radic Res. 2012 Mar;46(3):346-58. doi: 10.3109/10715762.2012.658514. Epub 2012 Feb 10. Free Radic Res. 2012. PMID: 22257113
Cited by
-
Development, characterization and use of rosemary essential oil loaded water-chestnut starch based nanoemulsion coatings for enhancing post-harvest quality of apples var. Golden delicious.Curr Res Food Sci. 2023 Aug 21;7:100570. doi: 10.1016/j.crfs.2023.100570. eCollection 2023. Curr Res Food Sci. 2023. PMID: 37701633 Free PMC article.
-
Electrochemical Sensors Based on the Electropolymerized Natural Phenolic Antioxidants and Their Analytical Application.Sensors (Basel). 2021 Dec 15;21(24):8385. doi: 10.3390/s21248385. Sensors (Basel). 2021. PMID: 34960482 Free PMC article. Review.
-
Chromatographic Methods Developed for the Quantification of Quercetin Extracted from Natural Sources: Systematic Review of Published Studies from 2018 to 2022.Molecules. 2023 Nov 22;28(23):7714. doi: 10.3390/molecules28237714. Molecules. 2023. PMID: 38067447 Free PMC article.
-
Implementation of an Analytical Method for Spectrophotometric Evaluation of Total Phenolic Content in Essential Oils.Molecules. 2022 Feb 16;27(4):1345. doi: 10.3390/molecules27041345. Molecules. 2022. PMID: 35209133 Free PMC article.
-
Influence of Cold Stress on Physiological and Phytochemical Characteristics and Secondary Metabolite Accumulation in Microclones of Juglans regia L.Int J Mol Sci. 2024 May 3;25(9):4991. doi: 10.3390/ijms25094991. Int J Mol Sci. 2024. PMID: 38732208 Free PMC article.
References
-
- Macheix J. J., Fleuriet A., Billot J. Fruit phenolics. CRC Press; Boca Raton, Florida, USA: 1990.
-
- Dixon R. A., Achnine L., Kota P., Liu C. J., Reddy M. S. S., Lang L. J. The phenylpropanoid pathway and the plant defence-A genomic perspective. Mol. Plant Pathol. 2002;3:371–390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

