Anti-inflammatory pretreatment enables an efficient dendritic cell-based immunotherapy against established tumors
- PMID: 17962945
- PMCID: PMC11030084
- DOI: 10.1007/s00262-007-0410-4
Anti-inflammatory pretreatment enables an efficient dendritic cell-based immunotherapy against established tumors
Abstract
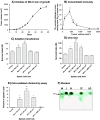
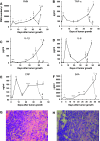
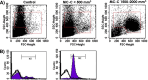
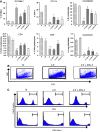
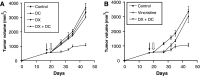
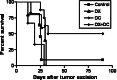
Although animals can be immunized against the growth of some tumor implants, most of the attempts to use immunotherapy to cause the regression of animal and human tumors once they have become established have been disappointing even when strongly immunogenic tumors were used as target. In this paper, we demonstrate that the failure to achieve an efficient immunological treatment against an established strongly immunogenic murine fibrosarcoma was paralleled with the emergence of a state of immunological unresponsiveness (immunological eclipse) against tumor antigens observed when the tumor surpassed the critical size of 500 mm(3). In turn, the onset of the immunological eclipse was coincidental with the onset of a systemic inflammatory condition characterized by a high number of circulating and splenic polymorphonucleated neutrophils (PMN) displaying activation and Gr1(+)Mac1(+) phenotype and an increasing serum concentration of the pro-inflammatory cytokines TNF-alpha, IL-1beta and IL-6 cytokines and C-reactive protein (CRP) and serum A amyloid (SAA) phase acute proteins. Treatment of tumor-bearing mice with a single low dose (0.75 mg/kg) of the synthetic corticoid dexamethasone (DX) significantly reduced all the systemic inflammatory parameters and simultaneously reversed the immunological eclipse, as evidenced by the restoration of specific T-cell-dependent concomitant immunity, ability of spleen cells to transfer anti-tumor activity and recovery of T-cell signal transduction molecules. Two other anti-inflammatory treatments by using indomethacin or dimeric TNF-alpha receptor, also partially reversed the immunological eclipse although the effect was not as striking as that observed with DX. The reversion of the immunological eclipse was not enough on its own to inhibit the primary growing tumor. However, when we used the two-step strategy of inoculating DX to reverse the eclipse and then dendritic cells loaded with tumor antigens (DC) as an immunization booster, a significant inhibition of the growth of both established tumors and remnant tumor cells after excision of large established tumors was observed, despite the fact that the vaccination alone (DC) had no effect or even enhanced tumor growth in certain circumstances. The two-step strategy of tumor immunotherapy that we present is based on the rationale that it is necessary to eliminate or ameliorate the immunological eclipse as a precondition to allow an otherwise ineffective anti-tumor immunological therapy to have a chance to be successful.
Figures
Similar articles
-
[Reversion of the immunological eclipse and therapeutic vaccination against cancer in an experimental model].Medicina (B Aires). 2007;67(1):44-8. Medicina (B Aires). 2007. PMID: 17408020 Spanish.
-
Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines.J Exp Med. 1996 Jan 1;183(1):87-97. doi: 10.1084/jem.183.1.87. J Exp Med. 1996. PMID: 8551248 Free PMC article.
-
Phenotypic profile of dendritic and T cells in the lymph node of Balb/C mice with breast cancer submitted to dendritic cells immunotherapy.Immunol Lett. 2016 Sep;177:25-37. doi: 10.1016/j.imlet.2016.07.009. Epub 2016 Jul 14. Immunol Lett. 2016. PMID: 27423825
-
Vaccination against human cancers (review).Int J Oncol. 2000 Jan;16(1):81-96. doi: 10.3892/ijo.16.1.81. Int J Oncol. 2000. PMID: 10601552 Review.
-
Dendritic cell-based vaccines for the therapy of experimental tumors.Immunotherapy. 2010 Mar;2(2):257-68. doi: 10.2217/imt.10.7. Immunotherapy. 2010. PMID: 20635932 Review.
Cited by
-
Inhibition of hyperprogressive cancer disease induced by immune-checkpoint blockade upon co-treatment with meta-tyrosine and p38 pathway inhibitor.BMC Cancer. 2022 Aug 3;22(1):845. doi: 10.1186/s12885-022-09941-2. BMC Cancer. 2022. PMID: 35922755 Free PMC article.
-
B cells inhibit the antitumor immunity against an established murine fibrosarcoma.Oncol Lett. 2017 May;13(5):3225-3232. doi: 10.3892/ol.2017.5810. Epub 2017 Mar 6. Oncol Lett. 2017. PMID: 28521429 Free PMC article.
-
Improvement of Antitumor Therapies Based on Vaccines and Immune-Checkpoint Inhibitors by Counteracting Tumor-Immunostimulation.Front Oncol. 2018 Jan 26;8:6. doi: 10.3389/fonc.2018.00006. eCollection 2018. Front Oncol. 2018. PMID: 29435437 Free PMC article.
-
Lymphadenectomy exacerbates tumor growth while lymphadenectomy plus the adoptive transfer of autologous cytotoxic cells and low-dose cyclophosphamide induces regression of an established murine fibrosarcoma.Cancer Immunol Immunother. 2011 Mar;60(3):389-99. doi: 10.1007/s00262-010-0949-3. Epub 2010 Dec 14. Cancer Immunol Immunother. 2011. PMID: 21153814 Free PMC article.
-
Analysis of pre-treatment markers predictive of treatment benefit for the therapeutic cancer vaccine MVA-5T4 (TroVax).Cancer Immunol Immunother. 2012 Dec;61(12):2283-94. doi: 10.1007/s00262-012-1302-9. Epub 2012 Jun 13. Cancer Immunol Immunother. 2012. PMID: 22692758 Free PMC article. Clinical Trial.
References
-
- Bustuoabad OD, Ruggiero RA, di Gianni P, Lombardi MG, Belli C, Camerano GV, Dran G, Schere-Levy C, Costa H, Isturiz MA, Narvaitz M, van Rooijen N, Bustuoabad VA, Meiss RP. Tumor transition zone: a putative new morphological and functional hallmark of tumor aggressiveness. Oncol Res. 2005;15:169–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

