Specifically targeted antiviral therapy for hepatitis C virus
- PMID: 17963291
- PMCID: PMC4171251
- DOI: 10.3748/wjg.v13.i43.5673
Specifically targeted antiviral therapy for hepatitis C virus
Abstract
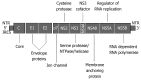
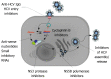
Hepatitis C virus (HCV) infection affects 180 million people worldwide with the predominant prevalence being infection with genotype 1, followed by genotypes 2 and 3. Standard anti-HCV therapy currently aims to enhance natural immune responses to the virus, whereas new therapeutic concepts directly target HCV RNA and viral enzymes or influence host-virus interactions. Novel treatment options now in development are focused on inhibitors of HCV-specific enzymes, NS3 protease and NS5B polymerase. These agents acting in concert represent the concept of specifically targeted antiviral therapy for HCV (STAT-C). STAT-C is an attractive strategy in which the main goal is to increase the effectiveness of antiviral responses across all genotypes, with shorter treatment duration and better tolerability. However, the emergence of resistant mutations that limit the use of these compounds in monotherapy complicates the regimens. Thus, a predictable scenario for HCV treatment in the future will be combinations of drugs with distinct mechanisms of action. For now, it seems that interferon will remain a fundamental component of any new anti-HCV therapeutic regimens in the near future; therefore, there is pressure to develop forms of interferon that are more effective, less toxic, and more convenient than pegylated interferon.
Figures
References
-
- Hepatitis C--global prevalence (update) Wkly Epidemiol Rec. 2000;75:18–19. - PubMed
-
- Barrera JM, Bruguera M, Ercilla MG, Gil C, Celis R, Gil MP, del Valle Onorato M, Rodés J, Ordinas A. Persistent hepatitis C viremia after acute self-limiting posttransfusion hepatitis C. Hepatology. 1995;21:639–644. - PubMed
-
- Alter HJ. HCV natural history: the retrospective and prospective in perspective. J Hepatol. 2005;43:550–552. - PubMed
-
- Appel N, Schaller T, Penin F, Bartenschlager R. From structure to function: new insights into hepatitis C virus RNA replication. J Biol Chem. 2006;281:9833–9836. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical